Research Article
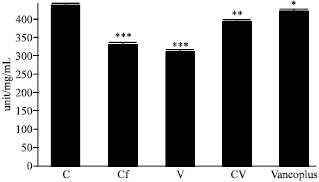
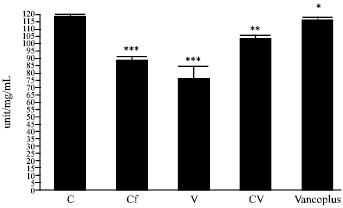
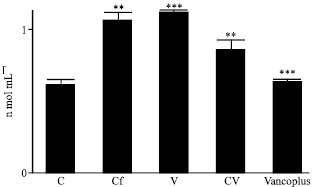
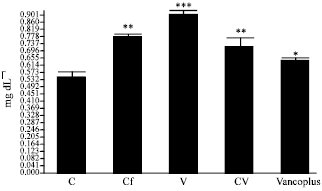
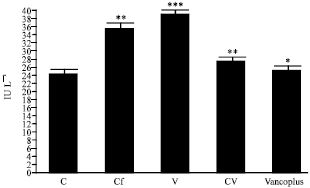
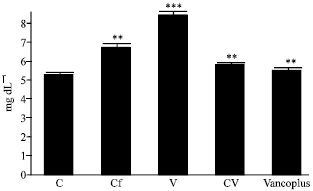
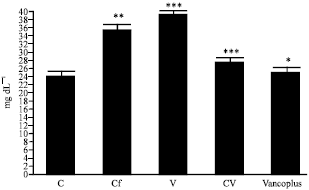
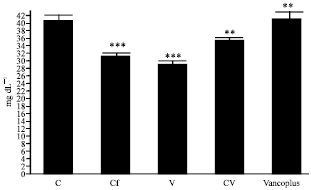
Nephrotoxicity Reduction by Ceftriaxone plus Vancomycin (Vancoplus) Reconstituted with VRP 1020 in Blood of Mus musculus Mice
Intellectual Scientific Division, Venus Medicine Research Centre, Hill Top Industrial Estate, Bhatoli Kalan, Baddi, HP-173205, India
Manu Chaudhary
Intellectual Scientific Division, Venus Medicine Research Centre, Hill Top Industrial Estate, Bhatoli Kalan, Baddi, HP-173205, India
Vivek Kumar Dwivedi
Intellectual Scientific Division, Venus Medicine Research Centre, Hill Top Industrial Estate, Bhatoli Kalan, Baddi, HP-173205, India