Research Article
Molecular Modelling Analysis of the Metabolism of Ambroxol
Discipline of Biomedical Science, School of Medical Sciences, Faculty of Medicine, Cumberland Campus, C42 The University of Sydney, Lidcombe, NSW, Australia
Ambroxol (trans-4-((2-amino-3,5-dibromobenzyl)aminocyclohexanol); AMB) is used to treat acute and chronic bronchitis, bronchiectasia, lung tuberculosis and coniosis, that may be co-administered with many types of drugs such as bronchodilators, steroids, antibiotics, expectorant and anti-allergic agents (Ishiguro et al., 2000). The drug also acts as an antioxidant (Gillissen et al., 1997). It is a mucolubricant that promotes secretion of surfactant and airway fluid, enhances ciliary movement and normalizes airway mucosa (Nagaoka and Kase, 1981). It is well absorbed and excreted in the urine about 50% as glucuronides of the unchanged drug, 10% as the oxidized metabolized 3,5-dibromo-2-aminobenzoic acid (DBABA) and a minute amount as cyclisation product 6,8-dibromo-3-(trans-4-hydroxycyclohexyl)-1,2,3,4-tetrahydroquinazoline (DHTQ) (Seki et al., 1977). Formation of DHTQ proceeds non-enzymatically whereas the formation of DBABA requires NADPH and the involvement of CYP3A4 (Ishiguro et al., 2000) that plays an important role in the metabolism of many drugs. Adverse side effects from AMB have been reported in patients taking several drugs that are metabolized by CYP3A4 (Honig et al., 1993). However, the potential for drug-drug interaction was suggested to be low for AMB (Seki et al., 1977).
In this study, molecular modelling analyses have been carried out using the program Spartan ’02 (2002) to investigate the relative stability of AMB and its metabolites DBABA and DHTQ with the aim of providing a better understanding of their relative toxicity. The study was carried out in the Discipline of Biomedical Science, School of Medical Sciences, The University of Sydney during the months of January 2007 to March 2007.
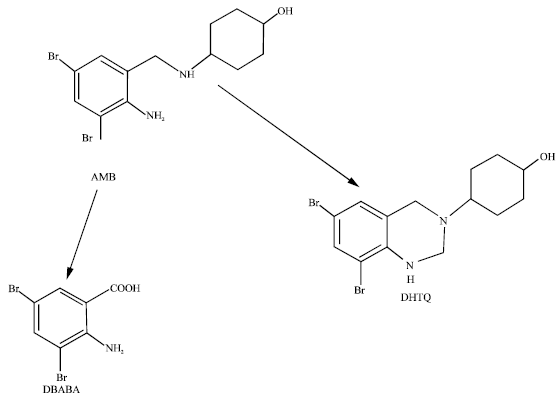 | |
| Fig. 1: | Metabolic pathways for AMB adapted from Ishiguro et al. (2000) |
COMPUTATIONAL METHODS
The geometries of AMB and its metabolites DBABA and DHTQ (Fig. 1) have been optimised based on molecular mechanics, semi-empirical and DFT calculations, using the molecular modelling program Spartan ’02 (2002). Molecular mechanics calculations were carried out using MMFF force field. Semi-empirical calculations were carried out using the routine PM3. DFT calculations were carried at B3LYP/6-31G* level. In optimization calculations, a RMS gradient of 0.001 was set as the terminating condition. Previous studies have shown that PM3 give more accurate results than MNDO and DFT (Density functional theory) calculations at B3LYP/6-31G* level give sufficiently reliable results (Huq, 2006). For the optimised structures, single point calculations were carried out to give heat of formation, enthalpy, entropy, free energy, dipole moment, solvation energy, energies for HOMO (Highest occupied molecular orbital) and LUMO (Lowest occupied molecular orbital). The order of calculations: molecular mechanics followed by semi-empirical followed by DFT ensured that the structure was not embedded in a local minimum. To further check whether the global minimum was reached, some calculations were carried out with improvable structures. It was found that when the stated order was followed, structure corresponding to the global minimum or close to that could ultimately be reached in all cases. Although RMS gradient of 0.001 may not be sufficiently low for vibrational analysis, it is believed to be sufficient for calculations associated with electronic energy levels.
Table 1 gives the total energy, heat of formation as per PM3 calculation, enthalpy, entropy, free energy, surface area, volume, dipole moment and energies of HOMO and LUMO as per both PM3 and DFT calculations for AMB and its metabolites DHTQ and DBABA.
| Table 1: | Calculated thermodynamic and other parameters of AMB and its metabolites |
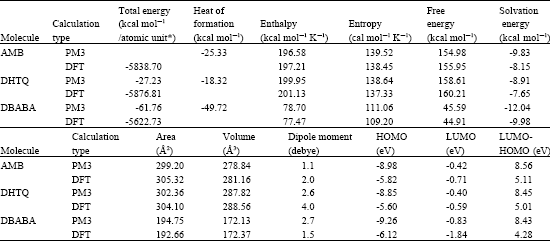 | |
| * in atomic units from DFT calculations | |
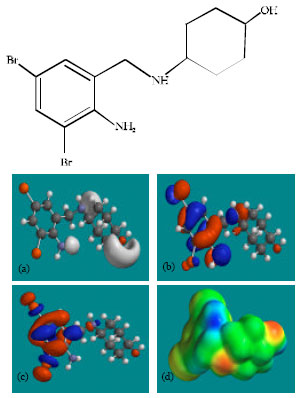 | |
| Fig. 2: | Structure of AMB giving in: (a) the electrostatic potential (greyish envelope denotes negative electrostatic potential), (b) the HOMOs, (where red indicates HOMOs with high electron density) (c) the LUMOs (where blue indicates LUMOs) and in (d) density of electrostatic potential on the molecular surface (where red indicates negative, blue indicates positive and green indicates neutral) |
Figure 2-4 give the regions of negative electrostatic potential (greyish-white envelopes) in (a), HOMOs (where red indicates HOMOs with high electron density) in (b), LUMOs in (c) and density of electrostatic potential on the molecular surface (where red indicates negative, blue indicates positive and green indicates neutral) in (d) as applied to the optimised structures of AMB and its metabolites DHTQ and DBABA.
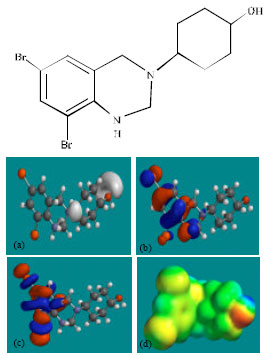 | |
| Fig. 3: | Structure of DHTQ giving in: (a) the electrostatic potential (greyish envelope denotes negative electrostatic potential), (b) the HOMOs, (where red indicates HOMOs with high electron density) (c) the LUMOs (where blue indicates LUMOs) and in (d) density of electrostatic potential on the molecular surface (where red indicates negative, blue indicates positive and green indicates neutral) |
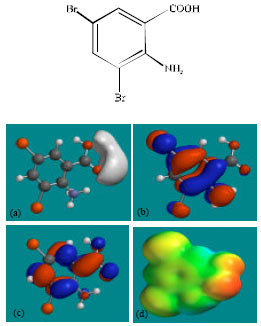 | |
| Fig. 4: | Structure of DBABA giving in: (a) the electrostatic potential (greyish envelope denotes negative electrostatic potential), (b) the HOMOs, (where red indicates HOMOs with high electron density) (c) the LUMOs (where blue indicates LUMOs) and in (d) density of electrostatic potential on the molecular surface (where red indicates negative, blue indicates positive and green indicates neutral) |
The calculated solvation energies from PM3 calculations of AMB and its metabolites DHTQ and DBABA are, respectively -9.83, -8.91 and -12.04 kcal mol-1 and the corresponding dipole moments from DFT calculations are 2.0, 4.0 and 1.5. The values suggest that DBABA would have the highest solubility in water and DHTQ the least.
The LUMO-HOMO energy differences for AMB and its metabolites DHTQ and DBABA from DFT calculations are found to be 5.1, 5.0 and 4.2 eV, respectively, indicating that AMB and its metabolites would be kinetically inert with DBABA being least inert.
In the case of AMB and DHTQ, the electrostatic potential is found to be more negative around the hydroxyl oxygen atom and the linking amino nitrogen atom, indicating that the positions may be subject to electrophilic attack.
In the case of DBABA, the electrostatic potential is found to be negative around the carboxyl oxygen atoms, indicating that the positions may be subject to electrophilic attack.
In the case of AMB, DHTQ and DBABA, both the HOMOs with high electron density and the LUMOs are found to be centred mostly on the non-hydrogen atoms of the aromatic ring.
The overlap of HOMO with high electron density and region of negative electrostatic potential at some positions, gives further support to the idea that the positions may be subject to electrophilic attack.
The molecular surfaces of AMB, DHTQ and DBABA, are found to abound in neutral (green) and electron-rich (yellow and red) regions so that they may be subject to lyophilic and nucleophilic attacks. The absence of significant amount of electron-deficient (blue) regions on the molecular surface means that the compounds may not readily with cellular nucleophiles such as glutathione and nucleobases in DNA. Thus, AMB and its metabolites may not induce cellular toxicity associated with glutathione depletion and may not cause DNA damage associated with oxidation of nucleobases.
Ambroxol (AMB) is used to treat acute and chronic bronchitis, bronchiectasia and lung tuberculosis and possesses antioxidant properties. Molecular modelling analyses based on molecular mechanics, semi-empirical (PM3) and DFT (at B3LYP/6-31G* level) calculations show that AMB and its metabolites DHTQ and DBABA have LUMO-HOMO energy differences of 5.11, 5.01 and 4.28 eV, respectively from DFT calculations. The molecular surfaces of AMB and its metabolites are not found to abound in electron-deficient regions so that the compounds may not react with glutathione and nucleobases in DNA. The presence of electron-rich regions on the molecular surface but absence of significant amount of electron-deficient regions, would impart onto AMB and its metabolites antioxidant properties.
Fazlul Huq is grateful to the Discipline of Biomedical Science, The University of Sydney for the time release from teaching.