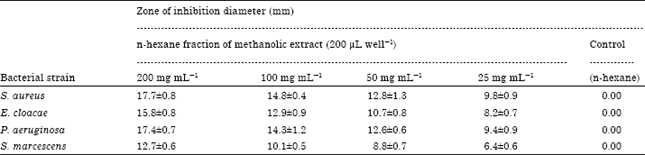
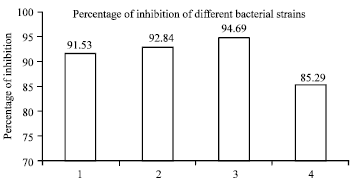
Research Article
In vitro Antibacterial Activity of n-Hexane Fraction of Methanolic Extract of Plumeria rubra L. (Apocynaceae) Stem Bark
Department of Botany, Presidency University, West Bengal, India
Trisha Das
Department of Zoology and Molecular Biology and Genetics, Presidency University, West Bengal, India
Souryadeep Mukherjee
Department of Zoology and Molecular Biology and Genetics, Presidency University, West Bengal, India