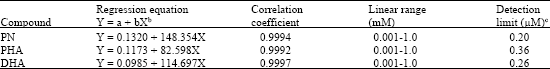Research Article
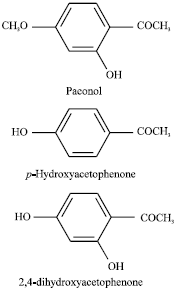
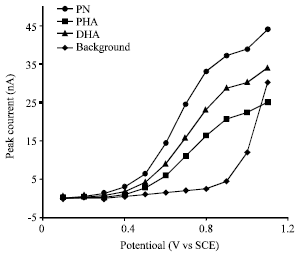
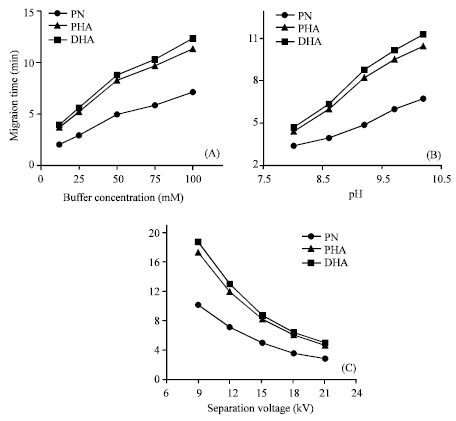
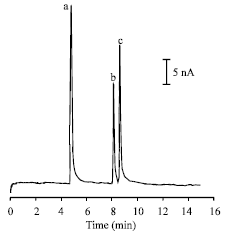
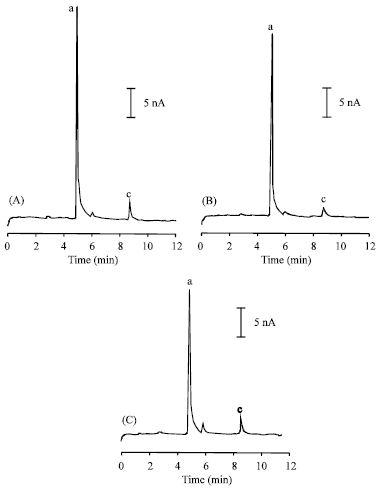
Determination of Phenolic Acetophenones in Radix Cynanchi Paniculati by Capillary Electrophoresis with Electrochemical Detection
Department of Chemistry, Fudan University, Shanghai 200433, China
Jianxia Zhang
Department of Chemistry, Fudan University, Shanghai 200433, China
Gang Chen
Department of Chemistry, Fudan University, Shanghai 200433, China