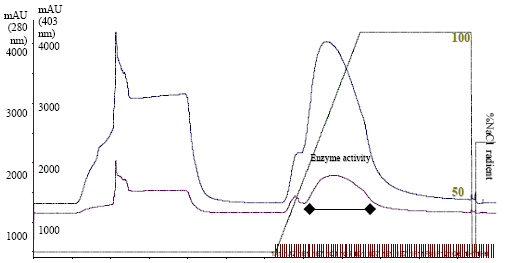
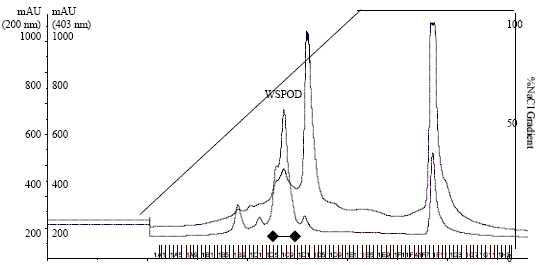
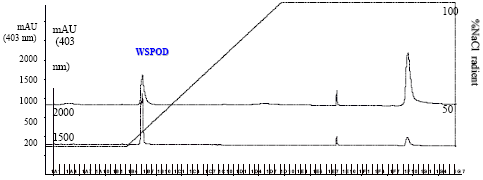
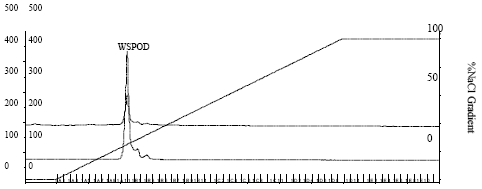
Research Article
A Novel Peroxidase from Withania somnifera
Division of Biotechnology and Division of Pharmacology, Regional Research Laboratory, Canal Road, Jammu-Tawi, 180 001, J and K, India
U. Jamwal
Division of Biotechnology and Division of Pharmacology, Regional Research Laboratory, Canal Road, Jammu-Tawi, 180 001, J and K, India
P. Kaiser
Division of Biotechnology and Division of Pharmacology, Regional Research Laboratory, Canal Road, Jammu-Tawi, 180 001, J and K, India
S. A. Tasduq
Division of Biotechnology and Division of Pharmacology, Regional Research Laboratory, Canal Road, Jammu-Tawi, 180 001, J and K, India
S. Rasool
Division of Biotechnology and Division of Pharmacology, Regional Research Laboratory, Canal Road, Jammu-Tawi, 180 001, J and K, India
V. Verma
Division of Biotechnology and Division of Pharmacology, Regional Research Laboratory, Canal Road, Jammu-Tawi, 180 001, J and K, India
G. N. Qazi
Division of Biotechnology and Division of Pharmacology, Regional Research Laboratory, Canal Road, Jammu-Tawi, 180 001, J and K, India