Research Article
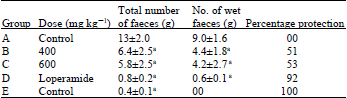
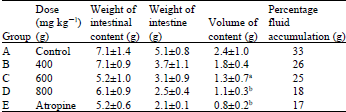
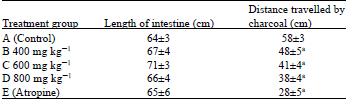
Evaluation of Antidiarrheal Efficacy of Detarium microcarpum Stem Bark Aqueous Extract in Albino Rats
Department of Veterinary Physiology, Pharmacology and Biochemistry, University of Maiduguri, P.M.B 1069, Borno State, Nigeria
A.A. Barkindo
Department of Animal Science and Rangeland Management, Modibbo Adama University, Yola, P.M.B 2076, Adamawa State, Nigeria
S. I. Ngulde
Department of Veterinary Physiology, Pharmacology and Biochemistry, University of Maiduguri, P.M.B 1069, Borno State, Nigeria
B. Wampana
Department of Veterinary Physiology, Pharmacology and Biochemistry, University of Maiduguri, P.M.B 1069, Borno State, Nigeria
K.A. Sanda
Department of Veterinary Physiology, Pharmacology and Biochemistry, University of Maiduguri, P.M.B 1069, Borno State, Nigeria