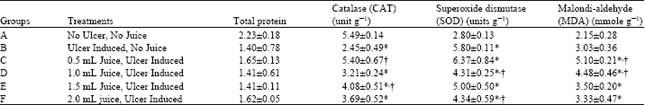
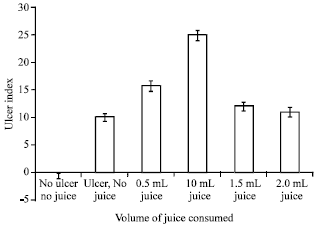
Research Article
Pineapple Juice Administration and Gastric Ulcer in Wistar Rats
College of Health Sciences, Faculty of Basic Medical Sciences (Remo Campus), Olabisi Onabanjo University, Ago Iwoye, Ogun State, Nigeria
T.O. Oyesola
College of Health Sciences, Faculty of Basic Medical Sciences (Remo Campus), Olabisi Onabanjo University, Ago Iwoye, Ogun State, Nigeria
A.I. Izagbo
College of Health Sciences, Faculty of Basic Medical Sciences (Remo Campus), Olabisi Onabanjo University, Ago Iwoye, Ogun State, Nigeria










Nancy plawecki Reply
I find your article very interesting. However, not plain enough for regular readers who don't have chem back grounds or lab. I love pineapple and have stomach issues. Suggestions,?