Research Article
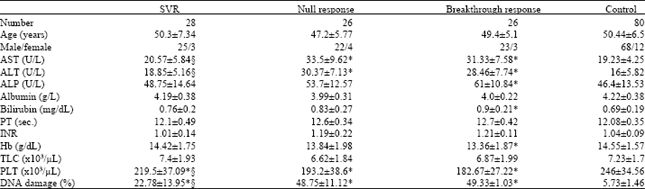
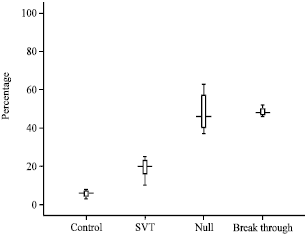
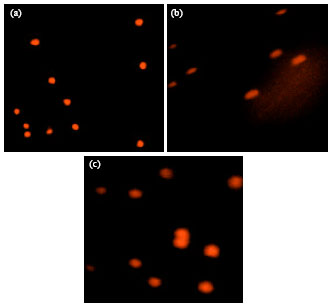
Peripheral Blood Lymphocytes' DNA Damage in Different Treatment Outcomes of Chronic Viral C Hepatitis Genotype 4 Infection
Department of Internal Medicine, Medical Research Division, National Research Centre, Cairo, Egypt
Safinaz El-Tokhy
Department of Medical Biochemistry, Medical Research Division, National Research Centre, Cairo, Egypt
Dalia El-Lebedy
Department of Clinical and Chemical Pathology, Medical Research Division, National Research Centre, Cairo, Egypt
Mohammed Abu Elfotouh
El-Tawfik Military Clinics, Cairo, Egypt
Ghada H. El-Arabi
Ministry of Health, Egypt