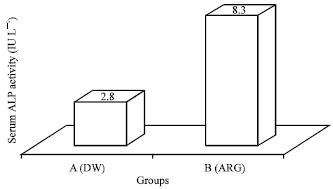
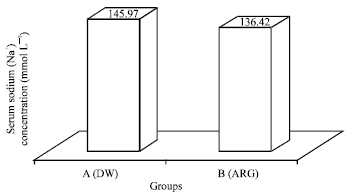
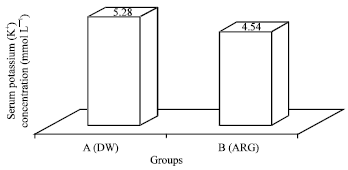
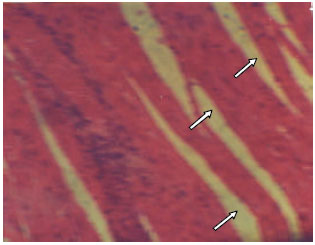
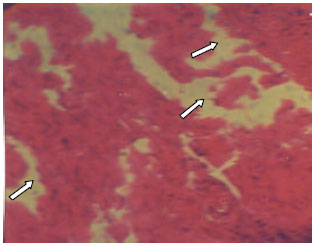
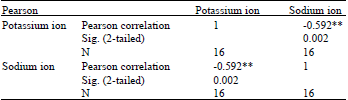
Research Article
Influence of L-arginine on the Heart Histology and Function Markers of Metabolic Syndrome in Female Wistar Albino Rats
Department of Biochemistry, University of Nigeria Nsukka, Enugu State, Nigeria
Ifeoma I. Ijeh
Department of Biochemistry, Michael Okpara University of Agriculture Umudike, Abia State, Nigeri
Lawrence U.S. Ezeanyika
Department of Biochemistry, University of Nigeria Nsukka, Enugu State, Nigeria
Onyechi O. Obidoa
Department of Biochemistry, University of Nigeria Nsukka, Enugu State, Nigeria