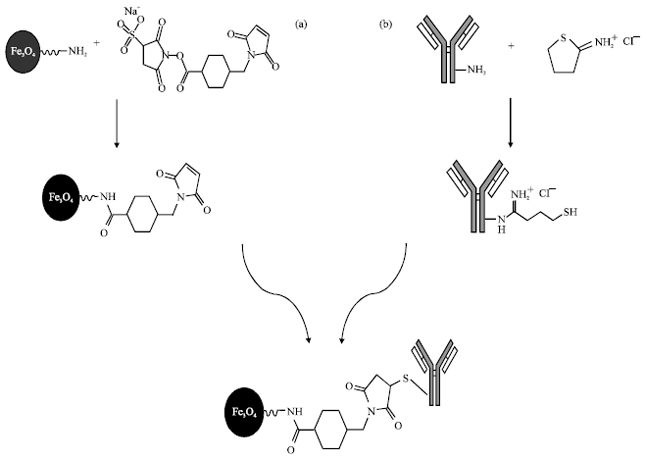
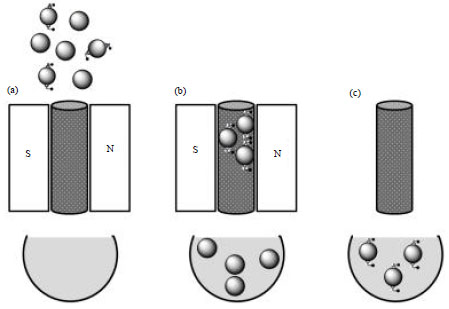
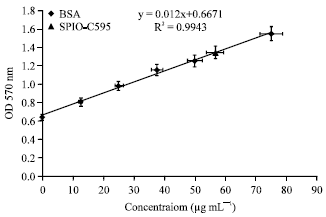
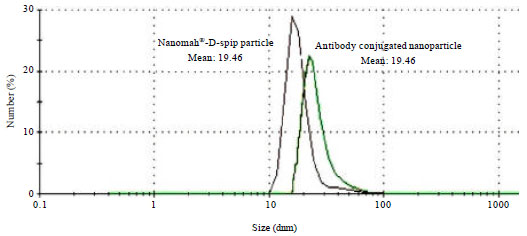
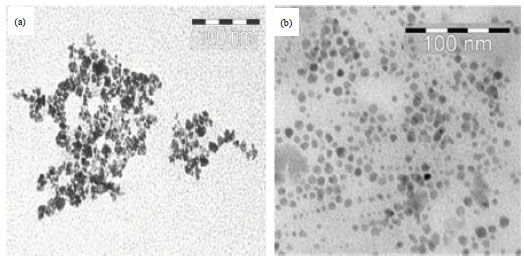
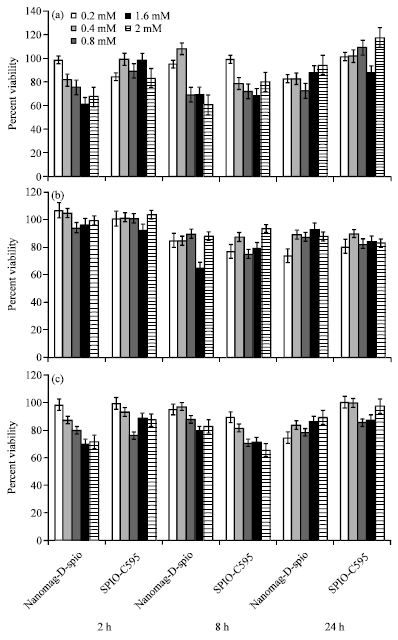
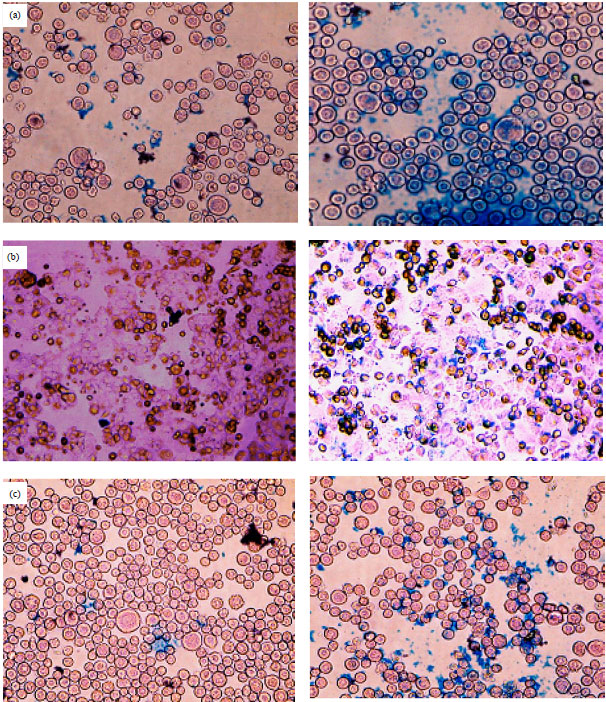
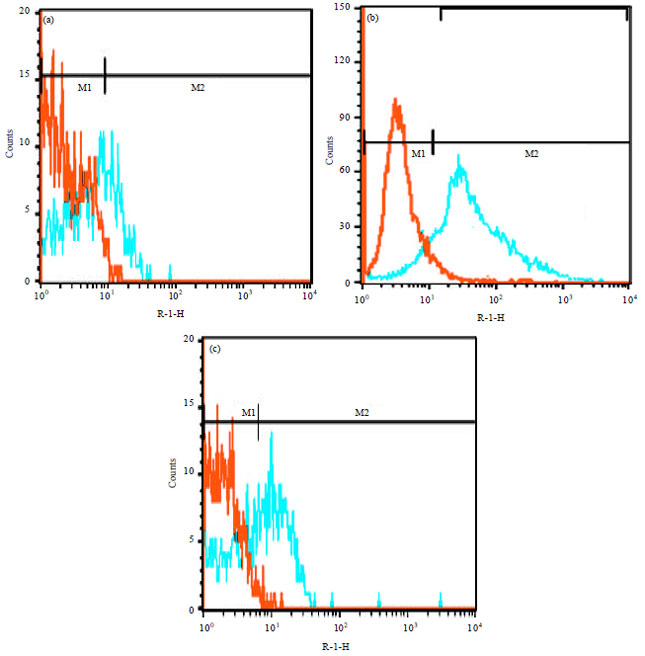
Research Article
A Novel Method for Quantitative Analysis of Anti-MUC1 Expressing Ovarian Cancer Cell Surface Based on Magnetic Cell Separation
Department of Medical Physics and Medical Engineering, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
Mohammad Abdolahi
Department of Medical Physics and Medical Engineering, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran