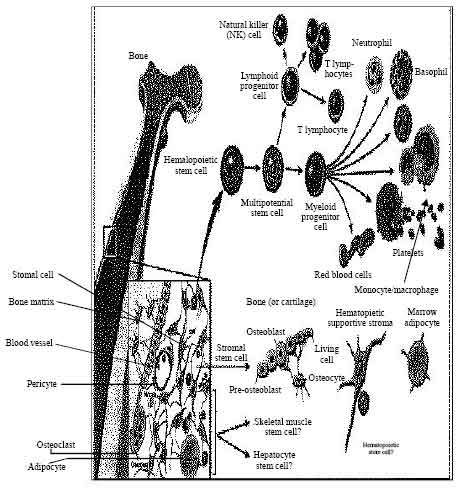
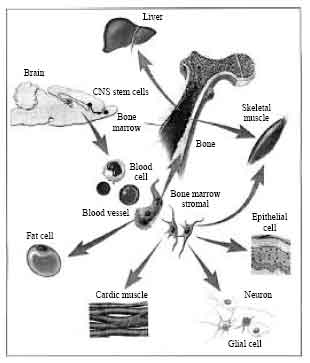
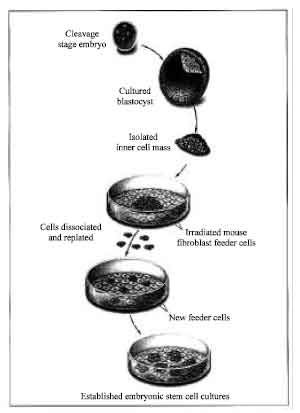
Review Article
New Vistas in the Therapeutic Uses of Stem Cells
Center for Molecular Medicine and Drug Research, International Center for Chemical Sciences, University of Karachi, Pakistan
Muhammad Ahmed Mesaik
Center for Molecular Medicine and Drug Research, International Center for Chemical Sciences, University of Karachi, Pakistan