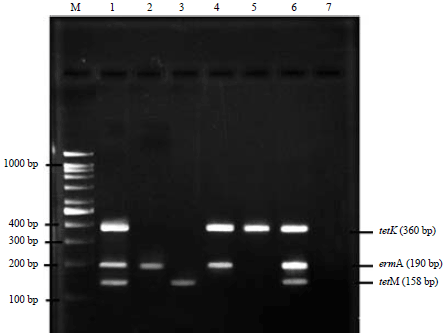
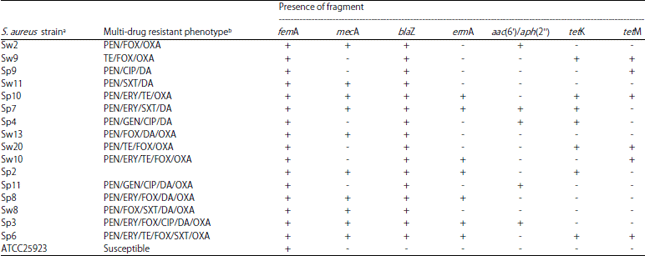
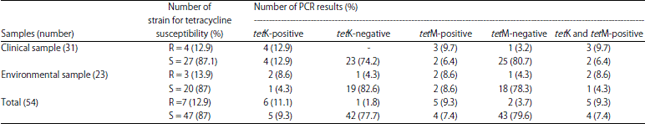
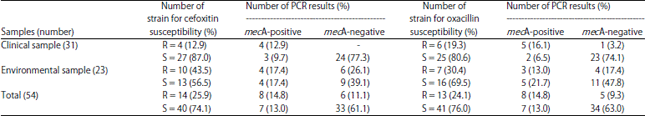Research Article
Antimicrobial Resistance Pattern of Staphylococcus aureus Strains Isolated from Clinical and Hospital Environment specimens and Their Correlation with PCR-based Approaches
Department of Microbiology, Faculty of Science, Silpakorn University, 73000 Nakorn Pathom, Thailand
LiveDNA: 66.12091
Nutthapol Mungchukeatsakul
Department of Microbiology, Faculty of Science, Silpakorn University, 73000 Nakorn Pathom, Thailand
Waya S. Phutdhawong
Department of Chemistry, Faculty of Science, Silpakorn University, 73000 Nakorn Pathom, Thailand