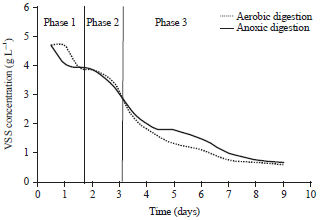
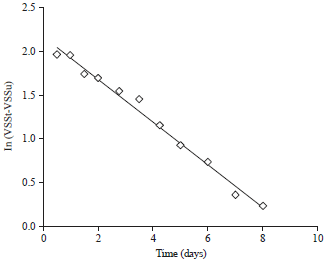
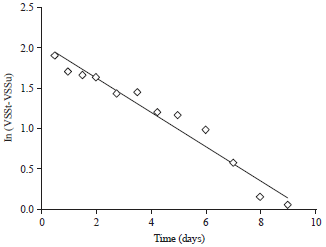
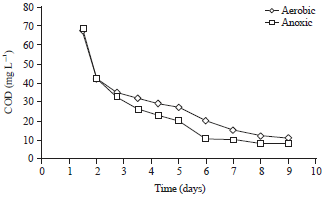
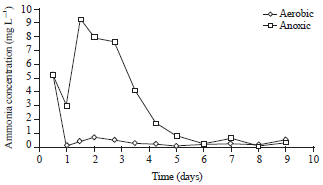
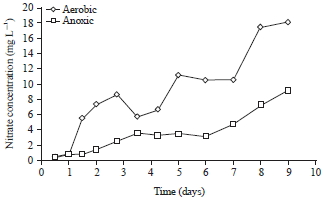
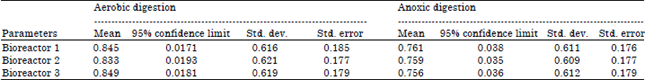
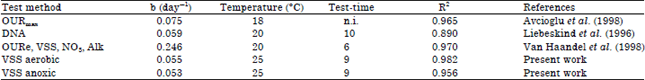
Research Article
Determination of Decay Coefficient of Biomass through Endogenous Respiration
Department of Civil and Environmental Engineering, Universiti Teknologi PETRONAS, 32160, Bandar Seri Iskandar, Perak, Malaysia
Shamsul R.B.M. Kutty
Department of Civil and Environmental Engineering, Universiti Teknologi PETRONAS, 32160, Bandar Seri Iskandar, Perak, Malaysia
Mohamed H. Isa
Department of Civil and Environmental Engineering, Universiti Teknologi PETRONAS, 32160, Bandar Seri Iskandar, Perak, Malaysia
Amirhossein Malakadmad
Department of Civil and Environmental Engineering, Universiti Teknologi PETRONAS, 32160, Bandar Seri Iskandar, Perak, Malaysia
Clement M. Ude
Department of Applied Biochemistry, Enugu State University of Science and Technology, 01660, Enugu State, Nigeria
Ezerie J. Menyechi
Department of Applied Microbiology and Brewery, Enugu State University of Science and Technology, 01660, Enugu State, Nigeria
Emmanuel Olisa
Department of Civil and Environmental Engineering, Universiti Teknologi PETRONAS, 32160, Bandar Seri Iskandar, Perak, Malaysia