Research Article
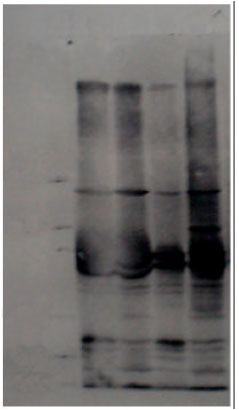
Antigenic Characterization of Mycoplasma agalactiae by SDS-PAGE and Immunoblotting
Department of Veterinary Microbiology, UP Pt. Deen Dayal Uphadhyaya Veterinary University and Gau Anusandhan Sanathan (DUVASU), Mathura, 281001 (UP), India
N.C. Srivastava
Division of Bacteriology and Mycology, Indian Veterinary Research Institute (IVRI), Izatnagar (UP), India
V.P. Singh
Division of Bacteriology and Mycology, Indian Veterinary Research Institute (IVRI), Izatnagar (UP), India