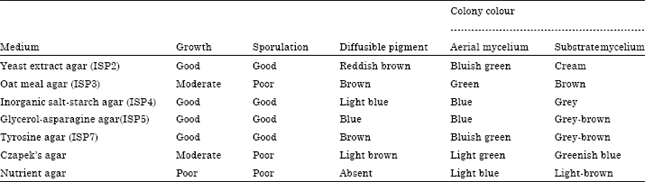
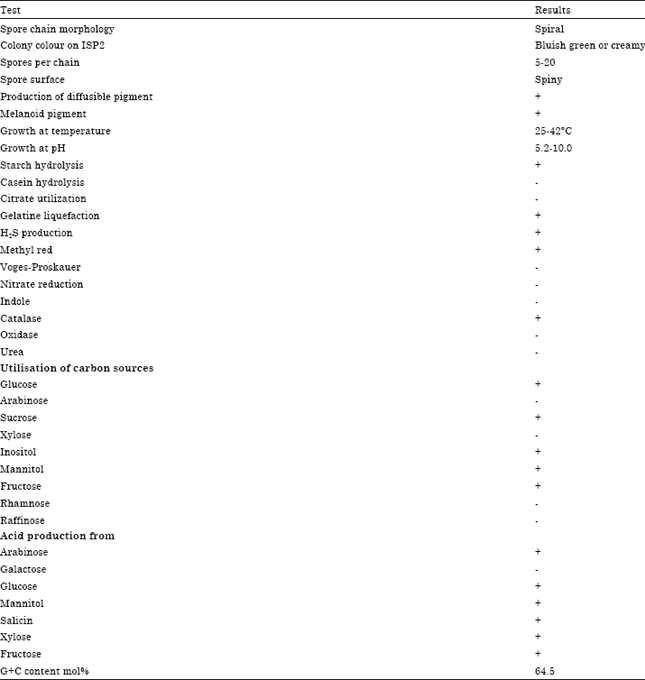
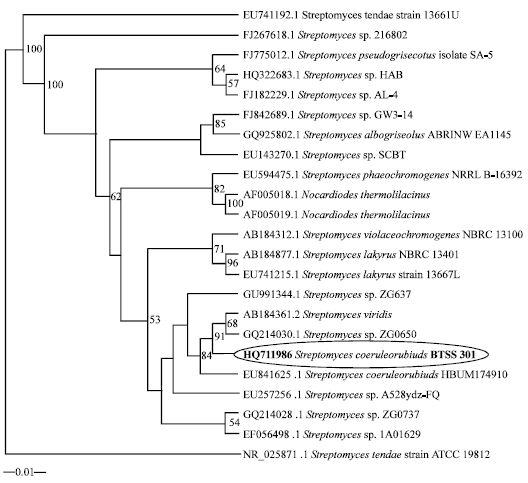
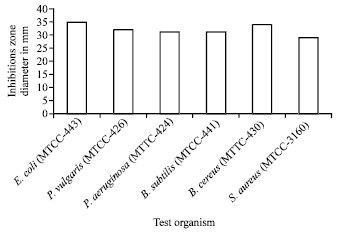
Research Article
Taxonomy and Antimicrobial Activity of Streptomyces coeruleorubidus sp. Isolated from Marine Sediment
Department of Biotechnology, College of Science and Technology andhra University, Visakhapatnam-530 003, Andhra Pradesh, India
R. Haritha
Department of Biotechnology, College of Science and Technology andhra University, Visakhapatnam-530 003, Andhra Pradesh, India
A. Swathi
Department of Biotechnology, College of Science and Technology andhra University, Visakhapatnam-530 003, Andhra Pradesh, India
B. Sirisha
Department of Biotechnology, College of Science and Technology andhra University, Visakhapatnam-530 003, Andhra Pradesh, India
T. Ramana
Department of Biotechnology, College of Science and Technology andhra University, Visakhapatnam-530 003, Andhra Pradesh, India