Research Article
Characterization of Alpha Amylase from Bacillus subtilis BS5 Isolated from Amitermes evuncifer Silvestri
Department of Microbiology, University of Ado-Ekiti, P.M.B. 5363, Ado-Ekiti, Nigeria
B.M. Olowe
Department of Microbiology, University of Ado-Ekiti, P.M.B. 5363, Ado-Ekiti, Nigeria









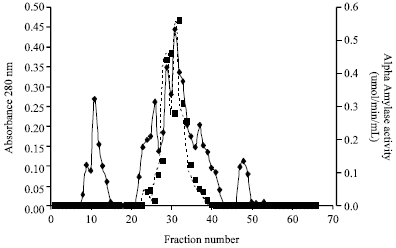
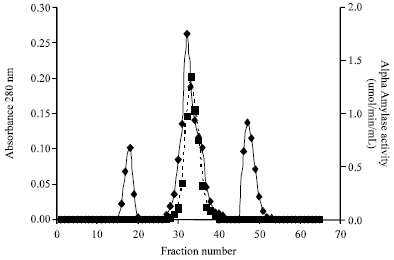
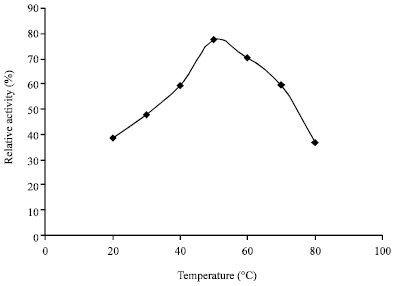

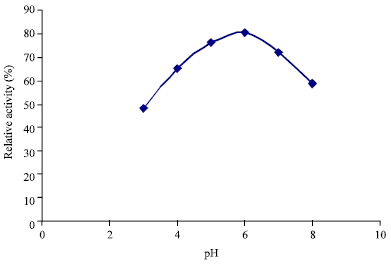


tanya tejeswita Reply
very clean results