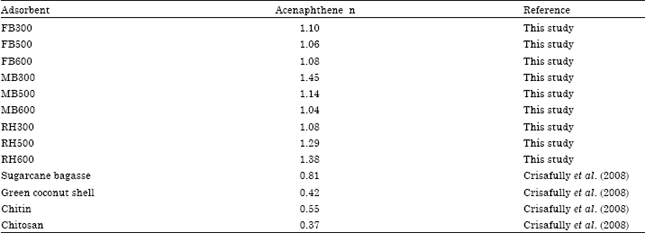
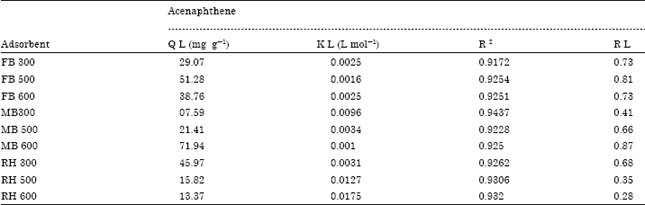
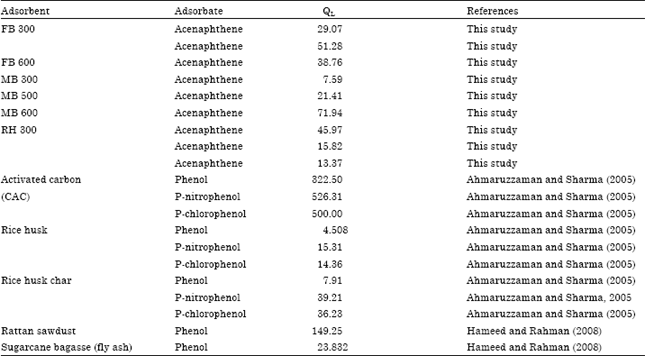
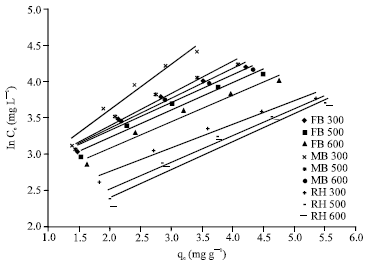
Research Article
Adsorption of Acenaphthene unto Activated Carbon Produced from Agricultural Wastes
Department of Chemical Engineering, Ladoke Akintola University of Technology, Ogbomoso, Nigeria
O.S. Amuda
Department of Pure and Applied Chemistry, Ladoke Akintola University of Technology, Ogbomoso, Nigeria
A.O. Afolabi
Department of Chemical Engineering, Ladoke Akintola University of Technology, Ogbomoso, Nigeria
F.E. Adelowo
Department of Chemical Engineering, Ladoke Akintola University of Technology, Ogbomoso, Nigeria