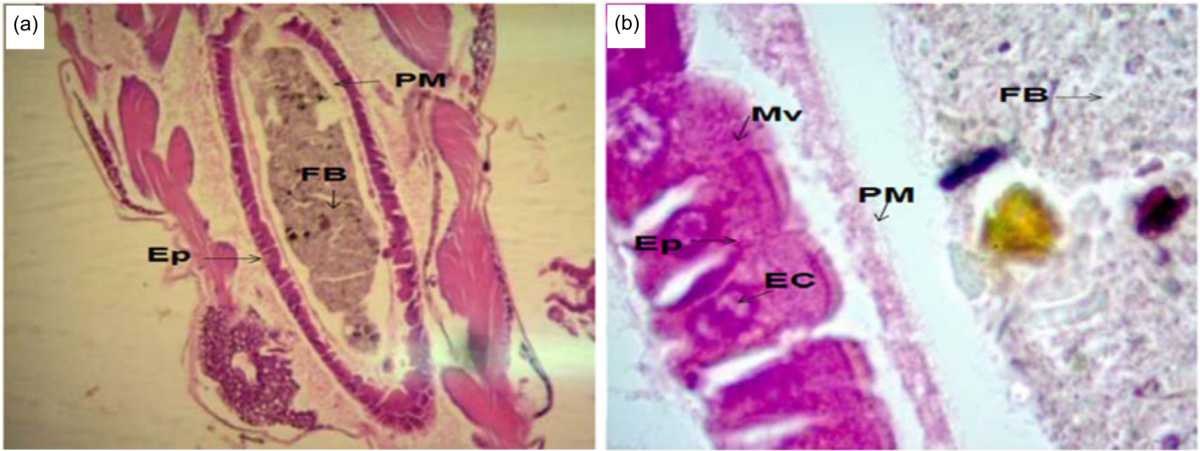
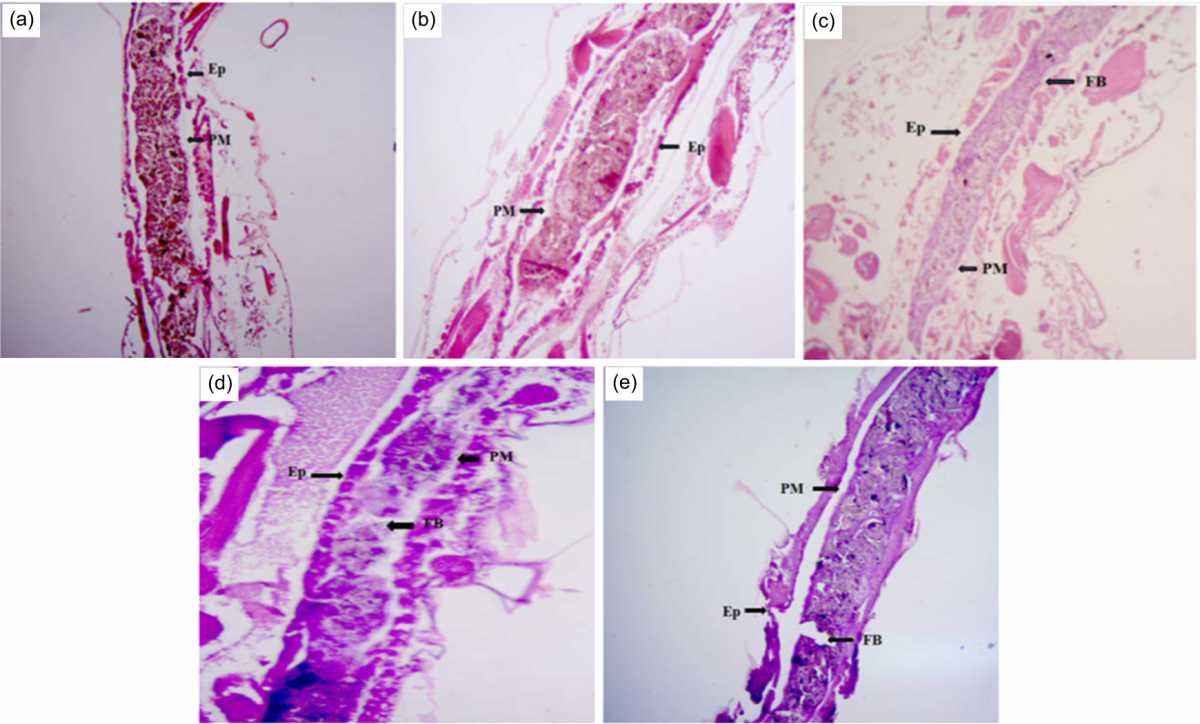
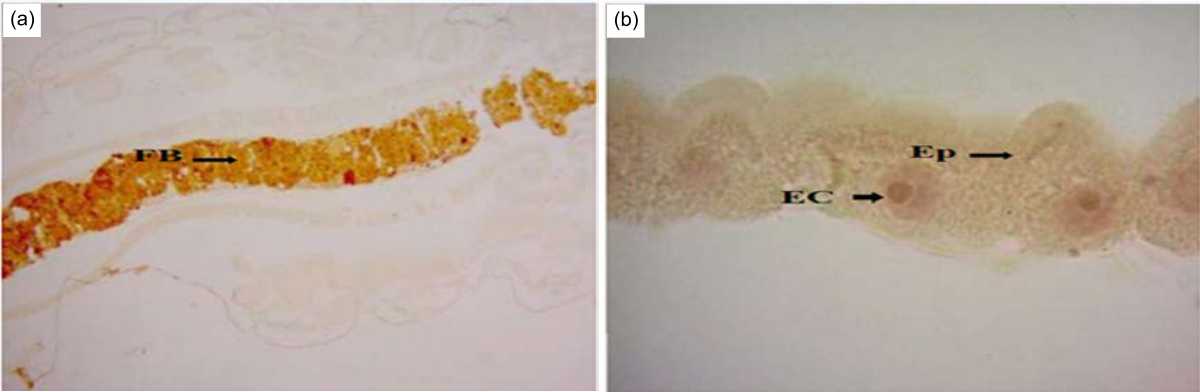
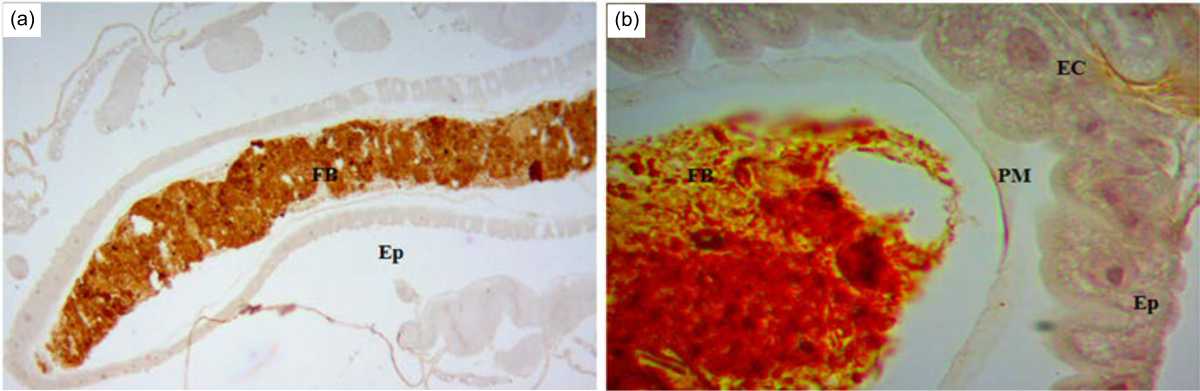
Research Article
Toxic Effects of Mentha piperita Extract on Culex quinquefasciatus Larvae (Diptera: Culicidae)
Department of Parasitology, Faculty of Medicine, University of Indonesia, Jl. Salemba Raya 6, Jakarta 10430, Indonesia
LiveDNA: 62.35053
Adib Kamil Putra Kadarusman
Department of Parasitology, Faculty of Medicine, University of Indonesia, Jl. Salemba Raya 6, Jakarta 10430, Indonesia
Fatmawaty
Department of Parasitology, Faculty of Medicine, University of Indonesia, Jl. Salemba Raya 6, Jakarta 10430, Indonesia
Nurhuda Sahar
Department of Parasitology, Faculty of Medicine, University of Indonesia, Jl. Salemba Raya 6, Jakarta 10430, Indonesia
Rawina Winita
Department of Parasitology, Faculty of Medicine, University of Indonesia, Jl. Salemba Raya 6, Jakarta 10430, Indonesia
Lisawati Susanto
Department of Parasitology, Faculty of Medicine, University of Indonesia, Jl. Salemba Raya 6, Jakarta 10430, Indonesia
Yulhasri
Department of Parasitology, Faculty of Medicine, University of Indonesia, Jl. Salemba Raya 6, Jakarta 10430, Indonesia
Nadar S. Lubis
Department of Parasitology, Faculty of Medicine, University of Indonesia, Jl. Salemba Raya 6, Jakarta 10430, Indonesia
Mulyati
Department of Parasitology, Faculty of Medicine, University of Indonesia, Jl. Salemba Raya 6, Jakarta 10430, Indonesia
Nurhadi Eko Firmansyah
Department of Parasitology, Faculty of Medicine, University of Indonesia, Jl. Salemba Raya 6, Jakarta 10430, Indonesia