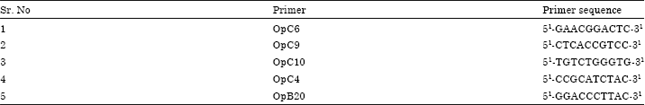
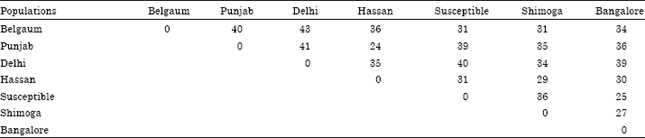
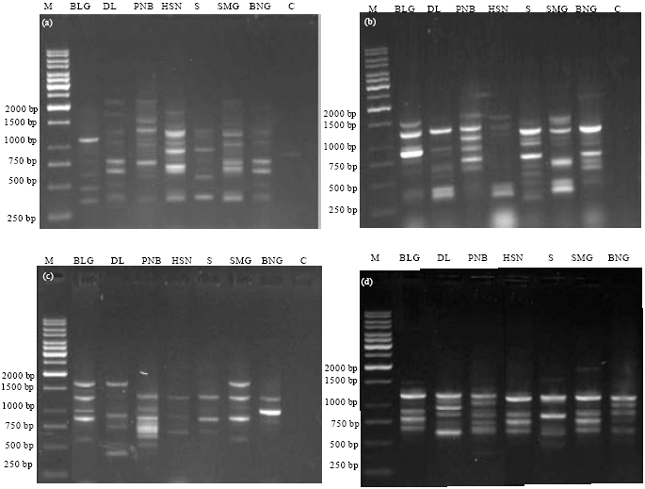
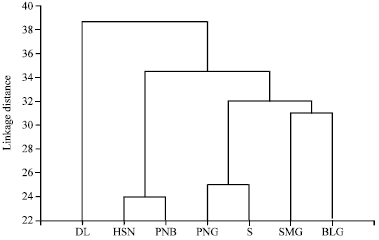
Research Article
Genetic Diversity of Diamondback Moth, Plutella xylostella L. (Yponomeutidae: Lepidoptera) Populations in India Using RAPD Markers
Department of Entomology, College of Agriculture, Bheemarayanagudi, 585 287, India
V. T. Sannaveerappanavar
Department of Entomology, University of Agricultural Sciences, GKVK, Bangalore, 560 065, India
K. S. Shankarappa
Department of Horticultural Plant Pathology, K.R.C. College of Horticulture, Arabhavi, 591 218, University of Horticultural Sciences, Bagalkot, Karnataka, India