Research Article
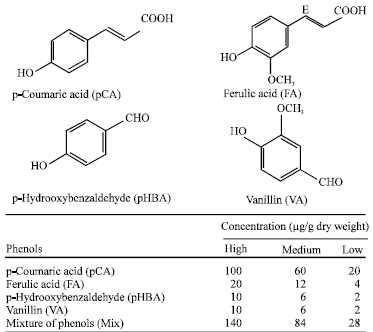
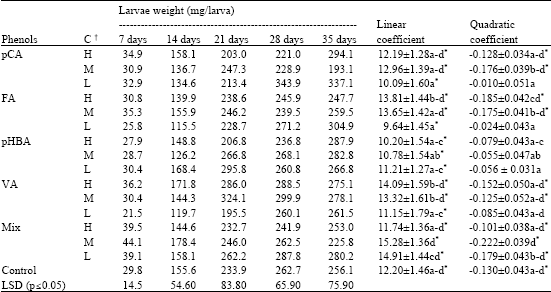
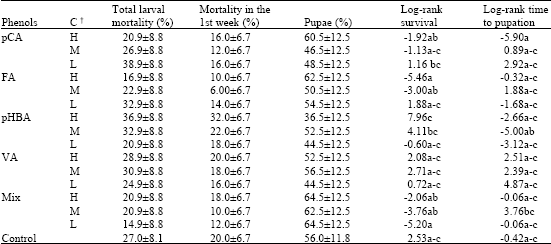
Effect of Maize Pith Free Phenols on Larval Growth and Development of Sesamia nonagrioides (Lepidoptera: Noctuidae)
Campus Universitario de Pontevedra, E-36005 Pontevedra, Spain
Xose C. Souto
Campus Universitario de Pontevedra, E-36005 Pontevedra, Spain
Liliana Monetti
Mision Biologica de Galicia. Spanish Cormcil for Scientific Research (CSIC), Apartado 28, E-36080 Pontevedra, Spain
Bernardo Ordas
Mision Biologica de Galicia. Spanish Cormcil for Scientific Research (CSIC), Apartado 28, E-36080 Pontevedra, Spain
Amando Ordas
Mision Biologica de Galicia. Spanish Cormcil for Scientific Research (CSIC), Apartado 28, E-36080 Pontevedra, Spain
Rosa A. Malvar
Mision Biologica de Galicia. Spanish Cormcil for Scientific Research (CSIC), Apartado 28, E-36080 Pontevedra, Spain