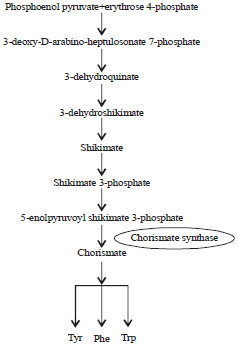
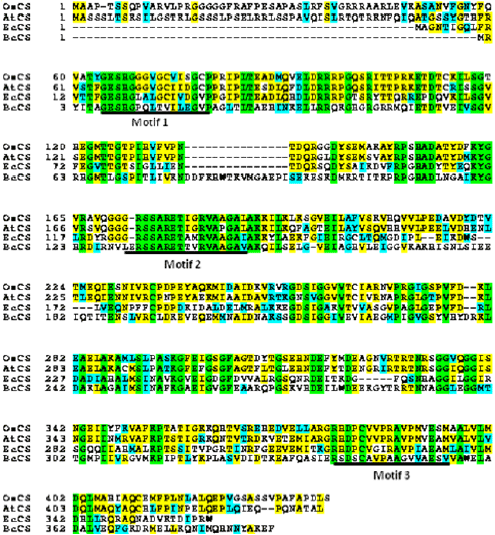
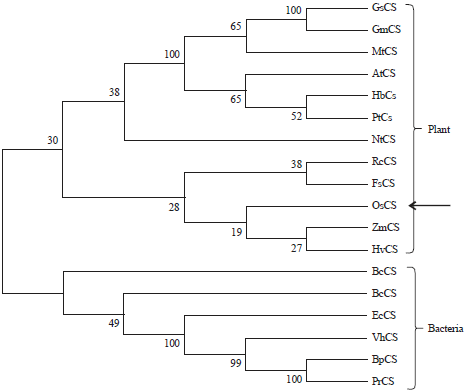
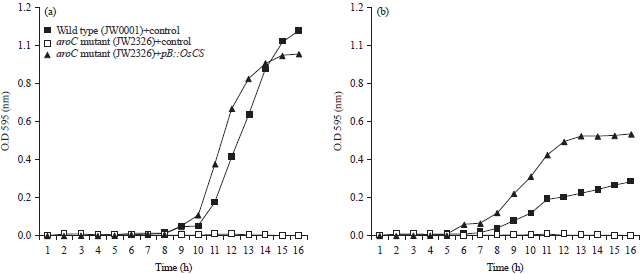
Research Article
Characterization and Functional Complementation with an Inhibitor of Rice Chorismate Synthase
Faculty of Biotechnology, Jeju National University, 690-756 Jeju, Republic of Korea
Md. Shafiqul Islam Sikdar
Department of Agronomy, Hajee Mohammad Danesh Science and Technology University, 5200 Dinajpur, Bangladesh
Jung-Sup Kim
Faculty of Biotechnology, Jeju National University, 690-756 Jeju, Republic of Korea