Research Article
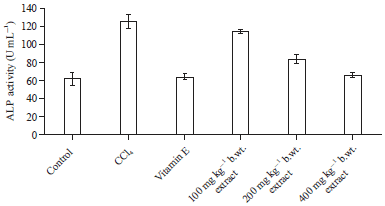
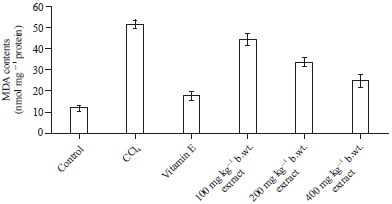
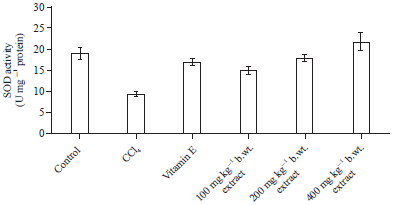
Effect of Methanol Extract of Abrus precatorius Leaves on Male Wistar Albino Rats Induced Liver Damage using Carbon Tetrachloride (CCl4)
Department of Biochemistry, University of Nigeria, Nsukka, Nigeria
Sangodare Rose Simon Adeyi
National Research Institute for Chemical Technology, Basawa Zaria, Kaduna State, Nigeria
Muhammad Kabir Hadiza
Nigeria Institute for Trypanosomiasis and Onchocerciasis Research (NITR), Kaduna State, Nigeria
Asadu Chidimma Lilian
Department of Biochemistry, University of Nigeria, Nsukka, Nigeria