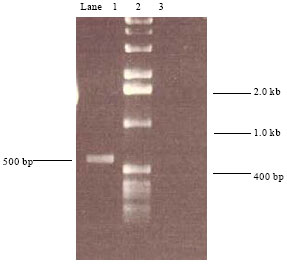
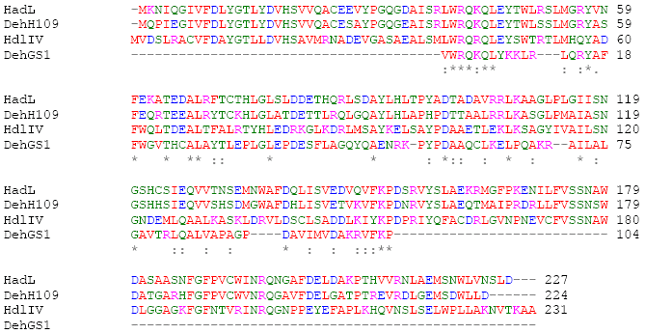
Research Article
Molecular Analysis of Dehalogenase Gene in Genomic DNA of Bacillus megaterium Strain GS1 Isolated from Volcanic Area Gunung Sibayak
Department of Industrial Biotechnology, Faculty of Biosciences and Bioengineering, Universiti Teknologi Malaysia, 81310 Skudai, Johor, Malaysia
D.D. Roslan
Department of Industrial Biotechnology, Faculty of Biosciences and Bioengineering, Universiti Teknologi Malaysia, 81310 Skudai, Johor, Malaysia
F. Huyop
Department of Industrial Biotechnology, Faculty of Biosciences and Bioengineering, Universiti Teknologi Malaysia, 81310 Skudai, Johor, Malaysia