Research Article
Effect of Salinity and Wytch Farm Crude Oil on Paspalum conjugatum Bergius (Sour Grass)
Department of Biology and Environmental Science, John Maynard Smith Building, University of Sussex, Falmer, Brighton, BN1 9QG, UK
The Niger Delta in the Southern region of Nigeria falls within the tropical rainforest zone: it is highly diverse and supportive of numerous species of terrestrial, aquatic flora and fauna and human life. These areas are, however, exposed to salinity. In the tropics saline soils are typified by lagoons and mangrove swamps and in some wet rainforest near the sea, toxic concentrations often occur in such soils because the soluble constituents are carried away in drainage water to the water table below the root range (Ewusie, 1980).
Not only is the Niger-Delta exposed to salinity, it is also the site of intense oil and gas exploration and production. Resources (oil and gas) from the region are the main source of revenue for Nigeria, accounting for about 97% of the country’s total export (Uyigue and Agho, 2007). Networks of pipelines run through not only coastal regions of the Niger Delta, but also through the rainforest, bush fallows and farmlands. Fifty percent of oil spills in Nigeria is due to corrosion of pipelines while 28% is due to sabotage (Badejo and Nwilo, 2004). Thousands of barrels of petroleum crude oil has been spilt into the environment through oil pipelines and tanks in the country. This loss is as a result of lack of regular maintenance of both pipelines and storage tanks. Little is known about the effect of petroleum pollution on plant species in the rainforest belt and less still of the effect of salinity on growth of these species (Agboola, 1998; Pezeshki et al., 2000).
The response of glycophytes (non-halophytes) to high salinity has been discussed in various reviews (Greenway and Munns, 1980; Gorham, 1992; Ehret and Plant, 1999; Parida and Das, 2005; Munns and Tester, 2008). In general, these reviews have shown that in glycophytes, increased soil salinity destroys hormonal equilibrium, reduces transpiration, respiration, water uptake, root growth and net photosynthesis. These results in low protein synthesis and causes dwarfed plant formation. Salinity also leads to reduction in biomass production (Parida and Das, 2005) number of flowers and reduction in yield (Osawa, 1963; Rush and Epstein, 1976; Levitt, 1980; Sharma, 1980; Robinson et al., 1983). Salinity can also cause affected plants to be stunted with dark green leaves, which, in some cases, are thicker and more succulent than normal and cause leaf burn and defoliation (Amacher et al., 1997; Munns, 2002).
On the other hand, oil spills have been known to cause acute and long-term damage to plants (Burns et al., 1993; Pezeshki and DeLaune, 1993; Qianxin and Mendelssohn, 1996; Pezeshki et al., 2000). These impacts range from reduction in population and growth rate or abnormal growth and regrowth after initial impact to plant mortality. The degree of oil impact also depends on various factors, such as the type and amount of oil, the extent of oil coverage, the plant species, the season of the spill, the soil composition and the flushing rate.
Paspalum conjugatum Bergius P.J. commonly known as sour grass, is a native grass of tropical America and a member of the Panicoideae-super tribe, Panicodae; tribe, Paniceae. It behaves both as an annual and perennial (Ismail et al., 1996) and rapidly invades wet habitats from sea level to 2000 m (Marie, 1965; Smith, 1985). Paspalum conjugatum is mostly glycophytic, mesophytic and a predominant species of open habitats (Watson and Dallwitz, 1992). It is naturalized throughout South-East Asia and in many tropical countries of the world. In Nigeria, it is commonly found in the rainforest belt, in farms, bush fallows and plantations of coconut and oil palm. Paspalum conjugatum is occasionally used as forage, lawn grass and is also regarded as an important weed in rice and plantation crops (Beetle, 1974). The little information available on this plant is concentrated on its uses, germination or eradication (Chee, 1990; Manidool, 1992; Ismail et al., 1985, 1990). Due to the occurrence of P. conjugatum in areas affected by periods of mild salinity and petroleum oiling and its invasive properties, it is hypothesized that P. conjugatum will not survive high salinity levels nor survive under the dual presence of salt and crude petroleum oil. To test these hypothesis P. conjugatum was grown in different soil salinity levels and two levels of oiling. Also the uptake of Na+ and K+ were measured to determine the combined effects of oil and salinity on mineral nutrition in P. conjugatum.
Plant stock culture and growth conditions: Paspalum conjugatum seeds were obtained from the Royal Botanical Gardens, Kew, Wakehurst Place, Ardingly in June 2004. P. conjugatum were initially grown outside as seeds were received in Summer and temperatures ranged between 22-27°C which was conducive for its growth. Seeds were pre-germinated in trays filled with Sinclair Horticulture Limited (SHL) professional all purpose compost, obtained from William Sinclair Horticulture Limited. Seeds were watered with tap water for 2 weeks, after which germinated seedlings were transplanted into 180 mm (diameter) x200 mm (height) pots filled with John Innes No. 2 soil and silver sand mix (ratio 1:3). In September 2004, plants were transferred into a greenhouse as day length was declining and temperatures cooling. Pots were placed in ebb and flow tanks (a flood and drain system) measuring 1000 mm (length)x1000 mm (breadth)x200 mm (depth). Culture solution used was Hoagland solution (Hougland and Arnon, 1950) and full strength contained (in mM) KNO3, 1.2; NH4H2PO4, 0.2; MgSO4, 0.4; and CaCl2, 1.0, together with micronutrients (in μM): H3BO3, 18.5; MnCl2, 3.7; ZnSO4, 0.3; CuSO4, 0.13; (NH4)6Mo7O24, 0.14 and FeNaEDTA, 113.
Fifty percent Hoagland solution was pumped into these tanks every 30 min, which were allowed to remain, flooded for 15 min before draining. The flood and drain action allowed for continuous aeration of the solution, which was changed every 2 weeks. The plants were illuminated with a minimum of 300 μmol/m/sec PAR, provided by supplemental lighting from 400-W HPI/T lamps and measured with a Licor-Quantum sensor (Li-185B) 30 cm above the pots, for up to 16 h light. Temperature ranged from 24-32°C during the day and 15-18°C at night. These conditions were maintained throughout all the experiments.
Experiments: Experiment 1, was conducted to investigate the effect of salt on growth of P. conjugatum. Full strength artificial seawater (Harvey, 1966) was prepared by adding the following salts (weights in grams of added salts per litre of solution for the equivalent of 100% seawater in parentheses): NaCl (4.98), Na2SO4 (3.91), CaCl2 (1.10) and KCl (0.66) to 50% Hoagland solution. In October 2004, after plants were left to acclimatize to greenhouse conditions for 30 days, 24 potted plants of P. conjugatum were selected from stock and sorted into six ASW treatments: 0, 7, 14, 29, 43 and 64% ASW (4 pots per treatment). Table 1 summarizes the concentration of Na+ and K+ ions in the various treatments. Solution was changed every 3 to 4 days to avoid a build up of salt in plant roots. Plants were harvested on day 21. Total chlorophyll concentration in leaves, dry weight and shoot Na+ concentration were analyzed.
In Experiment 2, the interaction of oil and salt on growth of P. conjugatum was studied. Wytch farm crude oil was obtained from Oil Spill Response Limited (OSRL) Southampton. It had a specific gravity (a measure of how heavy or light a petroleum liquid is compared to water) of 0.83 and classified as light crude oil. The amount of soil in pots was weighed. The pots 130 mm (height) x 120 mm (diameter) contained on average, 1,385 g soil. The rate of oil application was adapted from experiments carried out by Venosa et al. (2002). Application of 15 g of oil kg-1 soil was used as Light oiling (Lo) while 35 g of oil kg-1 soil was used for Heavy oiling (Ho). Oil was poured into soil at the various rates and mixed manually in plastic basins (while wearing seamless lined nitrile oil resistant hand gloves). Oil, at the various concentrations, was mixed thoroughly with the soil. Pots were filled with oiled soil before healthy rhizome cuttings (bearing shoots with an average of two leaves) of P. conjugatum (obtained from existing stock plants) were inserted into oiled or non-oiled potted soil: sand mix at the rate of 1 cutting per pot.
| Table 1: | Concentration of Na, K and Cl in ASW treatments for Paspalum conjugatum |
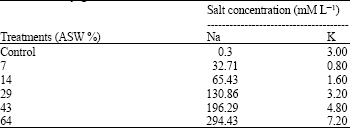 | |
| Control is fifty percent hoaglands | |
There were 6 treatments: Control (no oil, no ASW), Lo, Ho, Lo+ASW, Ho+ASW and ASW (4 pots per treatment). In saline treatments a constant saline level of 14% ASW was maintained. This salt level was chosen because results from experiment 1 showed that P. conjugatum survived at this salt level. At the end of the experimental period (21 days), visual morphological observations, plant height and number of leaves were recorded. P. conjugatum was harvested, separated into aboveground (shoots and leaves) and below ground (roots and rhizomes) components for determination of dry weight and ion concentration.
Dry weight determination: Aboveground (AG) and belowground (BG) were placed in brown paper bags and oven dried at 80°C for 48 h. Plant parts were weighed and accumulated biomass recorded.
Measurement of ion concentration: Each plant sample was grinded separately using a Glen Creston mill, which was cleaned after each sample. Ion concentration was measured following extraction of 10 mg of each powdered sample in 5 mL of 100 mM acetic acid in a water bath, maintained at a temperature of 80°C for 2 h. After 2 h, the solution was left to cool, filtered through Whatman No.1 (90 mm in diameter) filter paper and the filtrate analyzed for Na+ and K+ using a Unicam 919 atomic absorption spectrophotometer at a wavelength of 540 or 766.5 nm, respectively.
Total chlorophyll concentration: Total chlorophyll concentration was measured in 80% (v/v) acetone/water extracts by the method of Arnon (1949). In all the analysis, leaf discs were taken from fully expanded leaves of comparable physiological age, thereby eliminating developmental effects. Leaf discs weighing approximately 0.5 g were placed in volumetric flasks, 15 mL of 80% acetone was added, flasks stoppered, wrapped in foil and shaken vigorously for 20 sec. Flasks were placed in a cold room for 3 days. The color of the resultant supernatant ranged from dark green to pale yellow. The concentration of chlorophyll a and b were determined by measuring in a 10 mm cell, the optical density of 80% acetone chlorophyll extracts with a LKB Biochrom Ultrospec II spectrophotometer at 663 and 645 nm and setting up simultaneous equations using the specific absorption coefficients for chlorophyll a and b as given by MacKinney (1941). The spectrophotometer readings were converted into chlorophyll concentrations (mg chlorophyll g-1 fresh weight) using the following formula:
Statistical analysis: Results were analyzed statistically by using SPSS version 14 computer program, using one way analysis of variance (ANOVA), after conducting Levene’s test of homogeneity of variance. Significant differences between means (at the 95% level) were then determined using the Tukey HSD test (Field, 2000).
Response to increasing salinity: Increase in salinity of ASW resulted in a corresponding significant (p<0.05) decrease in dry weight of both AG and BG tissues of P. conjugatum (Fig. 1A, B). The adverse effect of artificial seawater became apparent from 14% ASW in AG dry weight and at 29% in BG dry weight. Dry weight continued to decrease significantly (p = 0.003) until mortality occurred between 43 and 64% ASW treatment confirming initial assumptions that P. conjugatum could not survive 100% ASW. In Fig. 2A, total chlorophyll concentration significantly decreased between 29 to 64% ASW treatments relative to Control. There was a 30% decrease in total chlorophyll in 29% ASW and in dead plants total chlorophyll concentration was significantly (p<0.05) decreased by 63 and 80% in 43 and 64% ASW treatments, respectively in comparison to control.
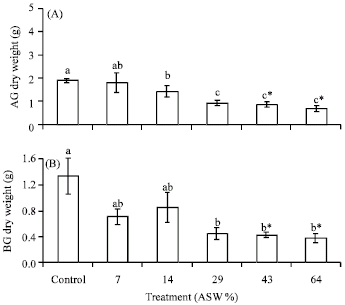 | |
| Fig. 1: | Effect of increase in salinity (ASW) on dry weight (g) of (A) Aboveground-AG and (B) Belowground-BG of Paspalum conjugatum. Plants were salinized with artificial seawater (ASW) at a concentration of 14% for 18 days. Values are the Means±SE for four replications. For each salinity level, means followed by different letters are significantly different at p<0.05 level (Tukey HSD). Letters marked by an *specify all plants died at indicated salinity. ASW = Artificial seawater |
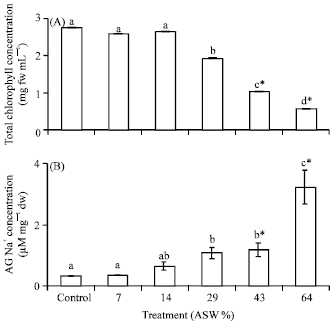 | |
| Fig. 2: | Effect of increase in salinity (ASW) on (A) total chlorophyll concentration (mg fresh weight mL-1) and (B) Aboveground Na+ concentration (μM mg-1 dw) of Paspalum conjugatum. Plants were salinized with artificial seawater (ASW) at a concentration of 14% for 21 days. Values are the Means±SE for four replications. For each salinity level, means followed by different letters are significantly different at p<0.05 level (Tukey HSD). Letters marked by an *specify all plants died at indicated salinity. ASW = Artificial seawater |
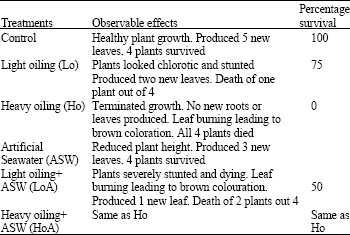
| Table 2: | Morphological changes observed in Paspalum conjugatum transplants under the individual and combined effect of Wytch farm crude oil and ASW for 21 days. n = 4 |
 | |
Increase in salinity resulted in a significant (p<0.001) increase in AG Na+ concentration to a maximum of 3.2 μM mg-1 dw in 64% ASW treatment compared to 0.63 μM mg-1 dw Na+ in 14% ASW treatment (Fig. 2B).
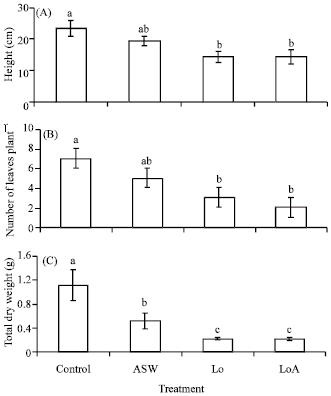 | |
| Fig. 3: | Effect of the individual and combined effect of ASW and Wytch farm crude oil on the A) height (cm), B) number of leaves (plant-1) and C) total dry weight (g) of Paspalum conjugatum transplants after 21 days. Plants were salinized with artificial seawater (ASW) at a concentration of 14%. Different letters above s.e bars indicate significant differences between means (n = 4). ASW = Artificial Seawater and Lo = Low oiling. Lo = 15 g oil kg-1 soil, Values for heavy oiling and heavy oiling+ASW were omitted as vegetative cuttings did not establish themselves in heavy oiled polluted soils |
Response to oiling and salinity: Recorded morphological observations for vegetative cuttings grown in saline and oiled soil for twenty-one days are shown in Table 2. In the absence of oil and salinity (Control), vegetative cuttings of P. conjugatum established and grew well. Seventy-five percent of vegetative cuttings grown in Lo soil survived while Ho was detrimental to the establishment of vegetative cuttings of P. conjugatum. Under the dual stress of Lo and 14% ASW (LoA) growth was significantly (p<0.05) impeded by reducing plant height, total number of leaves and total (AG + BG) dry weight of P. conjugatum (Fig. 3A-C). There was no data for height or total number of leaves in Ho and HoA treatments as cuttings did not establish and therefore died. Oiling significantly (p<0.005) decreased moisture content (%) and total chlorophyll concentration present in leaves (Fig. 4A, B).
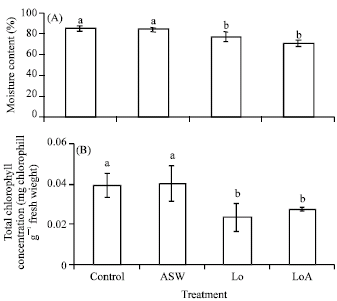 | |
| Fig. 4: | Effect of the individual and combined effect of ASW and Wytch farm crude oil on A) moisture content and B) total chlorophyll concentration (mg fw mL-1) of Paspalum conjugatum transplants exposed to the individual and combined effect of oiling for 21 days. Plants were salinized with Artificial Seawater (ASW) at a concentration of 14%. Different letters above s.e bars indicate significant differences between means (n = 4). ASW = Artificial Seawater, Lo = Low oiling, LoA = Low oiling+ASW |
As expected, plants grown under salinity had significantly (p<0.001) more Na+ than plants grown under Control and oiled-only treatments (Fig. 5A). Uptake of K+ was high in Control, ASW and Lo but significantly (p = 0.01) lower in LoA relative to Control (Fig. 5B). Individual treatments of ASW and oil caused no significant reduction in K+:Na+ ratio but a combination of the two (LoA) resulted in a significant (p<0.004) 73% decrease compared to Control (Fig. 5C).
Response to salinity: Paspalum conjugatum did not survive beyond 29% ASW. As there is no known published work on the effect of salinity on P. conjugatum, comparisons with other published literature is not possible. In this study, the sharp decrease in AG and BG dry weights of P. conjugatum as salinity increased indicated that this specie is capable of inhabiting saline environments of up to 29% ASW. The observed decrease in root dry weight of P. conjugatum with increasing salinity invariably led to an increased AG:BG weight ratio. If root growth is suppressed by salinity, nutrient uptake will be reduced and this may lead to poor growth overall. Though, Olusola and Okusanya (1990) reported low salt tolerance (10% seawater) in P. orbiculare found in the South West of Nigeria, Lee et al. (2004a, b) reported that P. vaginatum showed halophytic responses to salinity and some Paspalum ecotypes could tolerate up to sea water salinity.
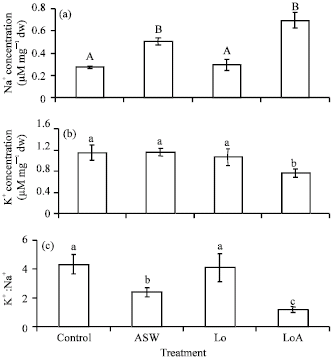 | |
| Fig. 5: | Effect of the individual and combined effect of ASW and Wytch farm crude oil on concentrations (μM mg-1 dw) of (A) Na+, (B) K+ and (C) K+:Na+ ratio of Paspalum conjugatum transplants grown for 21 days. Plants were salinized with artificial seawater (ASW) at a concentration of 14%. Different letters above s.e. bars indicate significant differences between means (n = 4) at p<0.05 level (Tukey HSD). ASW = Artificial Seawater, Lo = Low oiling and LoA = Low oiling+ASW. Lo = 15 g oil kg-1 soil. Values for heavy oiling and heavy oiling+ASW were omitted as vegetative cuttings did not establish themselves in heavy oiled polluted soil |
This is similar to results obtained for P. conjugatum in this study. Lee et al. (2005) showed that less salt tolerant Paspalum’s (for e.g., HI 34, SI 90 and Adalayd) exhibited a greater decrease in leaf water potential (Ψw) than salt tolerant ecotypes (SI 93-2 and HI 101).
Increase in salinity above 14% ASW resulted in a significant decrease in total chlorophyll concentration. A decrease in chlorophyll concentration would invariably mean a decrease in photosynthesis and this would result in a reduction of growth or plant death as observed in 43-64% ASW treatment. Furthermore, shoot Na+ concentration significantly increased with increase in salinity to a maximum of 3.2 μM mg-1 dw at 64% ASW.
Response to crude oil: Crude oil pollution has been shown to have adverse effects on plant growth and these may range from morphological aberrations and reduction in biomass to malfunction of gas exchange (Baker, 1971 a, b; Gill and Sandota, 1976; Sharma et al., 1980; Atuanya, 1987; Pezeshki et al., 2000; Meudec et al., 2007). The interaction of oil and salt on growth and mineral nutrition in P. conjugatum was studied in experiment 2. In the absence of salt, the application of oil at the rate of 15 g oil kg-1 soil (Lo) resulted in chlorotic leaves which could be attributed to decrease in total chlorophyll concentration (Fig. 4B). Despite a reduction in plant height, production of new leaves and total dry weight, plants established and survived. This is similar to the findings of Castro et al. (2006) who in assessing the toxicity of petroleum to stolons of Cynodon dactylon grown in soil containing different concentrations (0 to 200 g oil kg-1 soil) of Maya crude oil, reported chlorosis in leaves, decrease in number of leaves, nodes, shoot length and rhizome branches. On the other hand, heavy oiling (35 g oil kg-1 soil) caused death in rhizome cuttings of P. conjugatum. In this study, a relationship was observed between the extent of retardation of growth and concentration of crude oil applied to the soil. That is, light oiling had a less deleterious effect on the growth of P. conjugatum than heavy oiling. Similar to my results, Lin et al. (2002) demonstrated the dose-dependent effect of light oil (No. 2 fuel oil) level in soil on the growth of Spartina alterniflora. Moreover, Rosso et al. (2005) exposed Salicornia virginica to sediments polluted by two types of crude oils (the light ‘Escravos’ or the heavier Alba petroleum) and reported reductions in growth and photosynthesis. Also, Meudec et al. (2007) observed a decrease in viability of Salicornia fragilis (Glasswort) exposed to 2, 20 and 200 g oil kg-1 soil No. 2 fuel-oiled sediments for 6 to 12 weeks and noted that the decrease in viability depended on the amount of crude oil in the sediments.
In experiments, the presence of oil induced yellowing of leaves and necrosis (Table 2). Similarly, Alkio et al. (2005) reported chlorosis and necrosis because of localized H2O2 production, oxidative stress and cell death in Arabidopsis thaliana exposed to phenanthrene (a PAH component of crude oil). Meudec et al. (2007) also reported chlorosis and degeneration of tissues in Salicornia fragilis exposed to oiling. Analysis conducted on Wytch farm crude oil (Ibemesim, 2008) showed that it was rich in 2 and 3 ring hydrocarbons; for example in weathered WFC, naphthalene concentration was 8 μg L-1 while phenanthrene concentration was 3 μg L-1.
As might be expected from the yellowing of leaves, the chlorophyll concentration in P. conjugatum was grossly reduced with the addition of crude oil. A reduction in chlorophyll concentration has been used as an indicator for environmental pollution. Malallah et al. (1996) noticed a decrease in chlorophyll a and total chlorophyll content at optical densities of 435 and 415 nm for Vicia faba grown in various concentrations of oil polluted soils. Damage caused by oil may result from the chemical action of its compounds (heavy metals, Polycyclic aromatic compounds (PAHs) and alkylated PAHs). The PAHs are seen as priority pollutants by the United States Environmental Protection Agency (USEPA). Moreover, they have carcinogenic and/or mutagenic effects on animals, as well as phytotoxic properties (Hodgeson, 1990). In plants, they may impair physiological processes, inducing a gradual deterioration of plant metabolism and the disturbance of their development. For instance, benzo(a) pyrene affects plant photosynthesis and respiration by chlorophyll breakdown and inhibition of electron transport in Photosystem II (PSII) (Huang et al., 1996; Marwood et al., 2001).
The inability of rhizome cuttings of P. conjugatum to establish roots in heavily oiled soils may be as a result of the coagulatory effect of the oil on the soil, binding the soil particles into a water impregnable soil block which seriously impairs water drainage and oxygen diffusion (Gill and Nyawuame, 1989). Atuanya (1987) stated that seeds sown in such soil will fail to germinate. Chupakhina and Maslennikov (2004) reported that the emergence and growth of seedlings of Hordeum vulgare, Dactylis glomerata, Vicia sativa, Panicum miliaceum and Zea mays in 10% crude oil polluted soils was retarded by two to seven days. In 20% oil polluted soil, Meudec et al. (2007) recorded 90% mortality in Salicornia fragilis, which they attributed to the toxicity of the pollutant and the physical disturbances at the root level. They concluded that effects of oil on plants resulted from both physical effects and chemical toxicity. When mixed with soil/substratum, oil acts as a physical barrier restricting the oxygen movement into the soil, thus, preventing or reducing gas exchanges between roots and soil (Cowell and Baker, 1969; DeLaune et al., 1979). Accordingly, oil exacerbates the oxygen deprivation already present at the soil level in wetlands (Pezeshki et al., 2000). Furthermore, hydrophobic properties of petroleum reduce the wetability of oiled sediments and so affect the water and nutrient availability for plants (Amakiri and Onofeghara, 1984; Adam and Duncan, 2002). Thus, both the higher mortality and the growth inhibition of plants exposed to oiled sediments may be attributed, at least in part, to the soil anaerobic conditions and to the deficiency of water and nutrients.
In the absence of salt, Low oiling (Lo) had similar concentrations of Na+ and K+ compared to control.
However, the presence of oil in 14% ASW treatment (LoA) resulted in a significant decrease in K+ concentration (Fig. 5B) which invariably led to a significant decrease in K+, Na+ ratio.
This study investigated the tolerance of P. conjugatum salinity and oil tolerance of P. conjugatum. Paspalum conjugatum tolerated salinity up to 14% ASW and mortality occurred at 43% ASW. Results showed that though light pollution by Wytch farm crude oil caused a decrease in establishment and growth of P. conjugatum the survival rate was high whereas heavy oil pollution resulted in mortality. The interactive effect of oil and salt was detrimental to the growth of P. conjugatum and significantly decreased K+ concentration and K+:Na+ ratio. Due to results obtained in this study, the use of fungi as a bio remediating agent to aid the growth of P. conjugatum in low / heavy oiled contaminated soils will be the focus of future studies.
This study was funded by a scholarship from the Association of Commonwealth Universities.