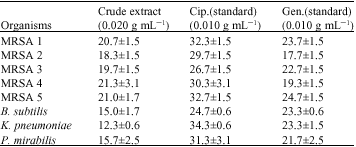
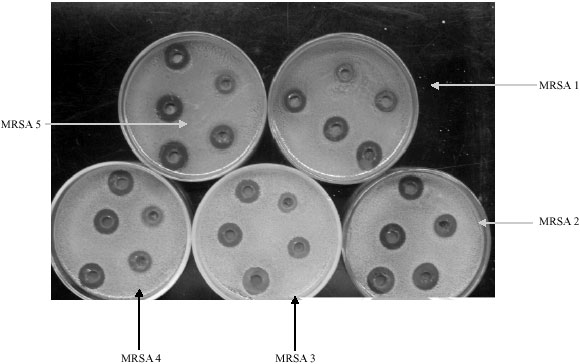
Research Article
Phytochemical Analysis and Antibacterial Activity of Khaya grandifoliola Stem Bark
Department of Basic Sciences, Faculty of Basic and Applied Sciences, Benson Idahosa University, Benin City, Edo State, Nigeria
F. Abiodun
Department of Pharmaceutical Chemistry,Faculty of Pharmacy, University of Benin, Benin City, Nigeria
O. Osahon
Department of Pharmaceutical Microbiology, Faculty of Pharmacy, University of Benin, Benin City, Nigeria
E. Ewaen
Department of Basic Sciences, Faculty of Basic and Applied Sciences, Benson Idahosa University, Benin City, Edo State, Nigeria