Research Article
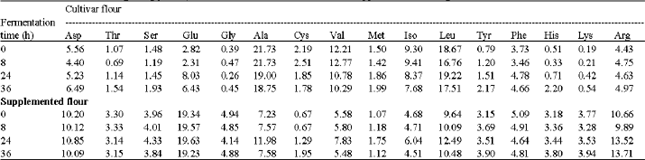
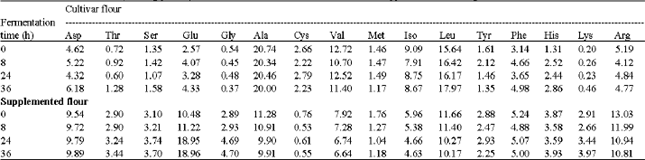
Biochemical Characteristics of Sorghum (Sorghum bicolor L. Moench) Flour Supplemented with Cluster Bean (Cyamopsis tetragonolaba L.): Influence of Fermentation and/or Cooking
Department of Biochemistry, Faculty of Science, University of Juba, Khartoum North, ElKadaro, Sudan
AbdelMoniem
Department of Food Science and Technology, Faculty of Agriculture, University of Khartoum, Khartoum North 13314, Shambat, Sudan
I. Mustafa
Department of Food Science and Technology, Faculty of Agriculture, University of Khartoum, Khartoum North 13314, Shambat, Sudan
Abdullahi H. El-Tinay
Department of Food Science and Technology, Faculty of Agriculture, University of Khartoum, Khartoum North 13314, Shambat, Sudan
Elfadil E. Babiker
Department of Food Science and Technology, Faculty of Agriculture, University of Khartoum, Khartoum North 13314, Shambat, Sudan