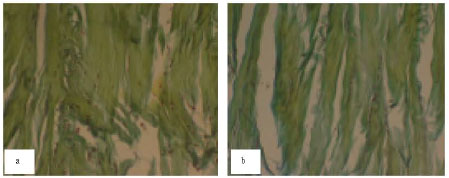
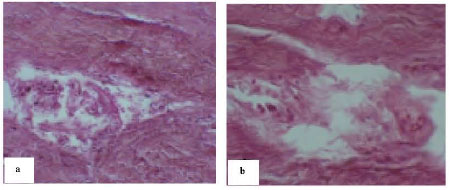
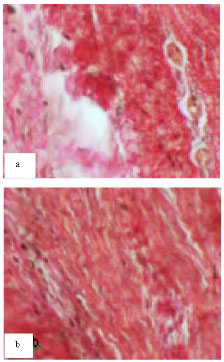
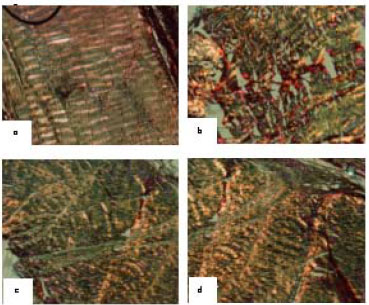
Research Article
Experimental Study of the Tendon Healing and Remodeling After Local Injection of Bone Marrow Myeloid Tissue in Rabbit
Department of Biology, School of Sciences, Ferdowsi University of Mashhad, Iran
Nasser Mahdavi Shahri
Department of Biology, School of Sciences, Ferdowsi University of Mashhad, Iran
Kamran Sardari
Department of Clinical Sciences, School of Veterinary Medicine, Ferdowsi University of Mashhad, Iran
Ali Moghimi
Department of Biology, School of Sciences, Ferdowsi University of Mashhad, Iran
Ahmad Reza Bahrami
Department of Biology, School of Sciences, Biotechnology and Tissue Engineering Research Center, Ferdowsi University of Mashhad, Mashhad, Iran
Masoumeh Kheirabadi
Department of Biology, School of Sciences, Ferdowsi University of Mashhad, Iran
Mohamad Amin Edalatmanesh
Department of Biology, School of Sciences, Biotechnology and Tissue Engineering Research Center, Ferdowsi University of Mashhad, Mashhad, Iran