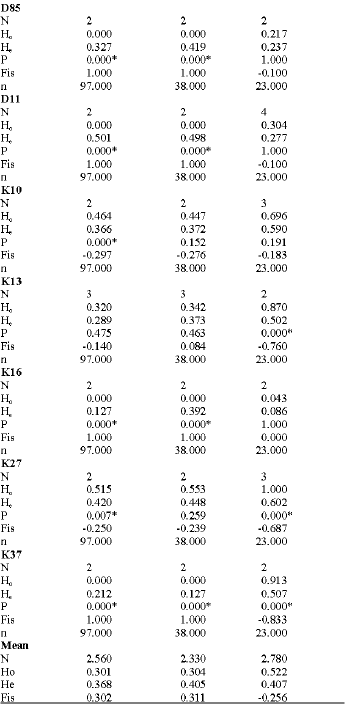
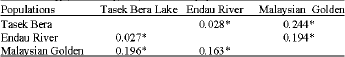
Research Article
Microsatellite Analysis of Wild and Captive Populations of Asian Arowana (Scleropages formosus) in Peninsular Malaysia
Bangladesh Fisheries Research Institute, Mymensingh-2201, Bangladesh
Mohd. Zakaria-Ismail
Institute of Biological Science, Faculty of Science, University of Malaya, 50603, Kuala Lumpur, Malaysia
Pek Yee Tang
Faculty of Engineering and Science, University Tunku Abdul Rahman, Malaysia
Sekaran Muniandy
Department of Molecular Medicine, Faculty of Medicine, University of Malaya, 50603, Kuala Lumpur, Malaysia