Research Article
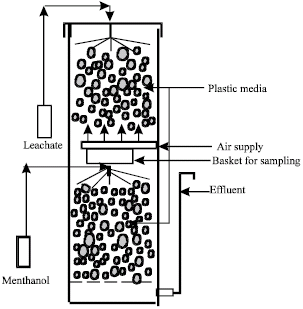
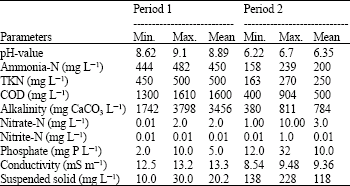
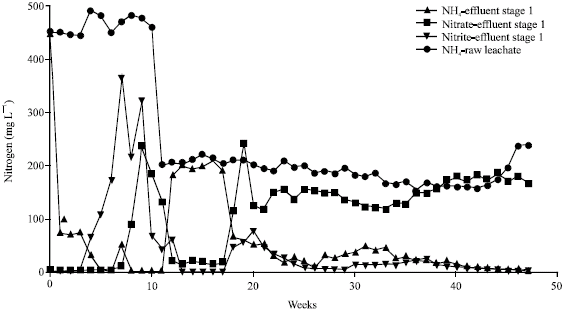
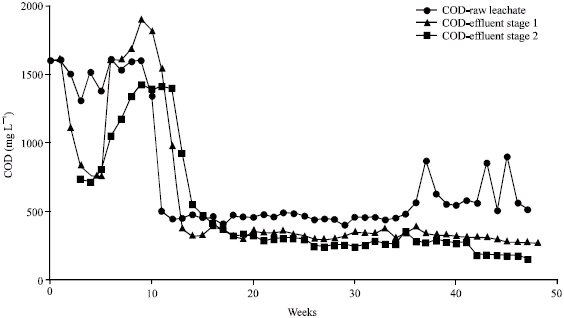
Removal of Ammonia from Landfill Leachate in a Two-Stage Biofiltration Process
Department of Environmental, Water and Earth Sciences, Tshwane University of Technology, Private Bag X680, Pretoria, 0001, South Africa
M.A.A. Coetzee
Department of Environmental, Water and Earth Sciences, Tshwane University of Technology, Private Bag X680, Pretoria, 0001, South Africa
S. Schwarzer
Department of Environmental, Water and Earth Sciences, Tshwane University of Technology, Private Bag X680, Pretoria, 0001, South Africa