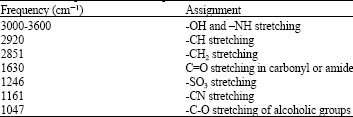
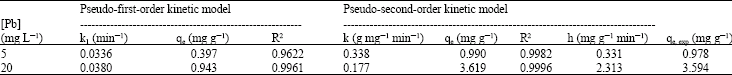
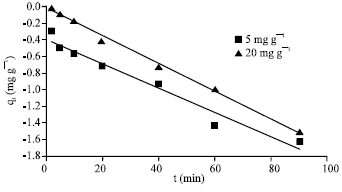
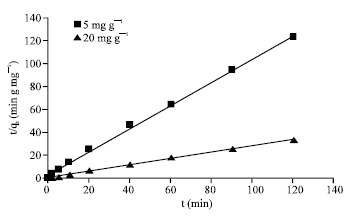
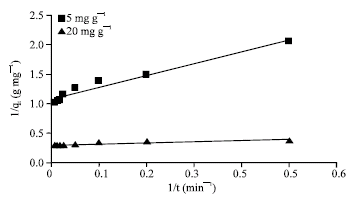
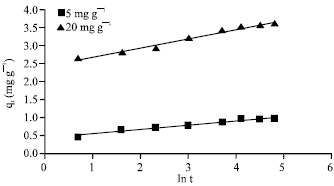
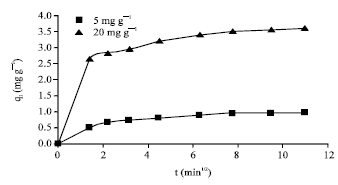
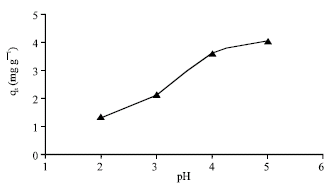
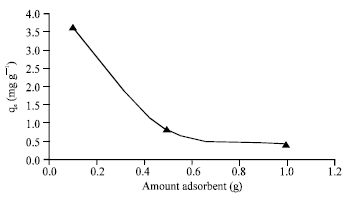
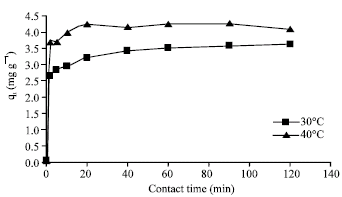
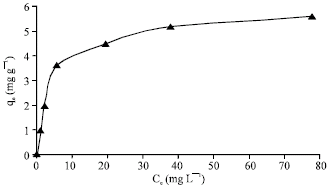
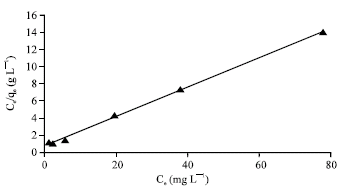
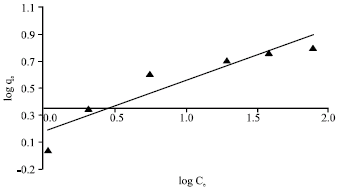
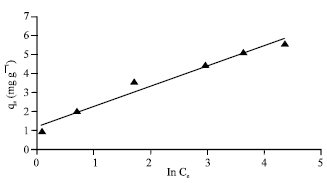
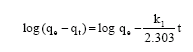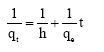Research Article
Batch Study of Liquid-Phase Adsorption of Lead Ions Using Lalang (Imperata cylindrica) Leaf Powder
Faculty of Applied Sciences, Universiti Teknologi MARA Pahang, 26400, Jengka, Pahang, Malaysia
W.S. Wan Ngah
School of Chemical Sciences, Universiti Sains Malaysia, 11800, Penang, Malaysia
H. Zakaria
Faculty of Applied Sciences, Universiti Teknologi MARA Pahang, 26400, Jengka, Pahang, Malaysia
S.C. Ibrahim
Faculty of Applied Sciences, Universiti Teknologi MARA, 40450, Shah Alam, Malaysia