Short Communication
Alteration in Morphological and Toxicological Properties of Copper Oxide Nano-particles: The pH Effect
Department of Zoology, Khalsa College, Amritsar 143001, Punjab, India
LiveDNA: 91.25755
In recent years, various novel nano-materials have received much attention due to their great potential for applications in agriculture, biomedical fields, food safety and food packaging1. Development of nano-particles is increasing due to their increasing wide applications in various fields including sensing technologies, sieving properties, electronics and biomedical applications2,3. As the manufacturing process of nano-particles is increasing day by day, their mixing in the environment is also at an upsurge. However, studies to assess their health risks and environmental impacts are still scarce. In different environmental conditions, these nano-particles undergo numerous physical and chemical changes which may change their nature and toxicity. Different studies report the toxic profiles of various types of nano-particles4-6. The impact of physical and chemical modifications of nano-particles on their biological function is a critical issue. Due to large surface area, nano-particles pose greater toxicities as compared to their larger counterparts. Nano-particles may enter the human body through different routes including skin, oral route or inhalation7. This entry of nano-particles into the human body may lead to toxicity in the form of protein misfolding, DNA damage, mitochondrial damage, membrane damage or production of reactive oxygen species.
Copper oxide (CuO) is the simplest member in the family of copper compounds possessing potential physical properties, such as high temperature superconductivity, photovoltaic, as an electrode in batteries and gas sensing. Nano-biotechnological application of copper nano-particles has also paved the way for advancement in agriculture owing to its bactericidal and fungicidal activities8. As a catalyst, nano-crystalline CuO is highly efficient in carbon monoxide oxidation. Various recent studies are being done on CuO nano-particles in different fields of science9-14. Copper is one of indispensable elements for maintaining homeostasis in various types of organisms and in ionic form it may lead to a situation to cause toxicity once they exceed the physiological tolerance range in vivo. Therefore, with the advancement in the nanotechnology, the investigation on the possible health effects and toxicology of CuO nano-particles is of great importance in scientific community. The CuO nano-particles serve several important functions in human life, particularly in the fields of medicine, engineering and technology. Further use for CuO nano-particles has been employed in the pharmaceutical industry especially in the production of anti-microbial fabric treatments or prevention of infections caused by Escherichia coli and methicillin-resistant Staphylococcus aureus7. Different models have been employed in recent researches on CuO nano-particles including Oryza sativa15, Pisum sativum16, mice11,17, Mytilus edulis18 and human blood7. The CuO nano-particle cytotoxicity was found associated with significant increase in intracellular reactive oxygen species level, loss of mitochondrial membrane potential and lysosomal membrane leakiness7. Hence, there is an urgent need to investigate more about toxicological aspects of CuO nano-particles. Thus in this study, the toxicity of CuO synthesized at different pH of the precursor solutions (pH 7 and 10) using Allium cepa root analysis was investigated. This study will surely add to the existing knowledge about the effect of pH on morphological as well as toxicological aspects of CuO nano-particles.
The present study was conducted at the Department of Zoology, Khalsa College Amritsar. The study was conducted for 2 months from July up to August, 2018.
Copper oxide nano-particles: The CuO nano-particles were procured in the powder form from Department of Physics, Khalsa College Amritsar, Punjab, India, which were synthesized from cupric nitrate tri-hydrate (Cu(NO3)2.3H2O) and citric acid monohydrate (C6H8O7.H2O) at pH7 and 10 of the precursor solution. The CuO powder samples were designated as NP1 and NP2 corresponding to sample synthesized at pH value 7 and 10, respectively.
Characterization of nano-particles
X-Ray diffraction analysis: The phase identification of the powder samples for pure CuO phase was performed by X-ray diffraction (XRD) on a X’PertPanlytical diffractometer using Cu Kα radiation (λ = 1.5405 Å, 30 mA , 40 kV) in 2θ range from 30-80°.
FESEM and TEM analysis: The surface topography of CuO powder samples was studied by scanning electron micrographs taken using JEOL JSM-6700F with a beam voltage of 30 KV. The TEM images were taken using transmission electron microscope system (HRTEM, model FEI Technai 30) operated at 300 KV.
Treatment sample preparation: Three concentration groups per CuO powder samples (NP1 and NP2) were prepared and annotated as per the Table 1.
| Table 1: | Formulation of different treatment concentrations of CuO nano-particles solutions |
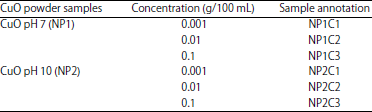 | |
Allium cepa root test
Test material: Onion bulbs (Allium cepa L.) of average size (15-20 mm diameter) were purchased from the local market. The onion bulbs were sun-dried for 5 weeks. The roots of the dried bulbs were cut off from the base with a sharp blade. This exposed the fresh meristematic tissues and the bulbs were placed in distilled water to protect the primordials from drying up.
Treatment of test material: After removing excess water with a blotting paper, the bases of all the onion bulbs were dipped in tap water for 5 days which resulted in a normal root growth. After these initial growth days, the onion bulbs were dipped in solutions of all the test solutions as described in Table 1. A series of seven onion bulbs were used for each sample concentration and control (tap water). The experiment was run for 7 days in dark. After the exposure time is over, out of the 7 exposed onion bulbs, best 5 onions in terms of root length development were chosen for analysis.
Root length measurement: After the exposure period, five selected onion bulbs were taken for the root length measurement. The root length (cm) of all onion bulbs was measured on 3rd and 7th day using a calibrated ruler taking the average of five longest roots. After taking the root lengths, the mean was calculated for each concentration treatment. The mean root length of the control samples was also calculated.
Statistical analysis: The difference between mean root lengths in the treatment groups and control was analyzed using Mann-Whitney U-test. Mean and standard error values were found using descriptive analysis and p<0.05 was considered as the significant level of the statistical analysis. All statistical analyses were performed using the program Minitab version 16.1.0 (Minitab Inc.) for windows.
XRD analysis: The XRD diffractograms of the CuO samples (NP1 and NP2) were obtained. The diffractogram of the samples revealed the polycrystalline nature of the samples. Among the various diffraction peaks, two prominent highly intense peaks of 002 and 111 atomic planes of CuO shows the probable grain growth direction and reveals most stable, minimum energy growth phase of crystal. No other peak corresponding to any undesired phase of Cu or Cu2O has been noticed.
The crystallite size in various samples of CuO was evaluated using the full width at half maximum β (FWHM) from most prominent (002) peak by using the Scherrer’s formula as:
| (1) |
where, λ = 1.5405, Å for Cu Kα radiations and θ is the Bragg angle.
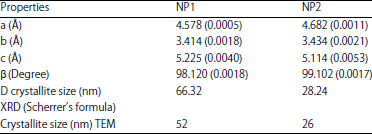
The values of lattice parameters calculated for both the nano-particle samples are tabulated in Table 2. The crystallite size in samples was found to be less (28.24 nm) in case of pH10 precursor solution (NP2) as compared to NP1 (66.32 nm). Thus increase in pH of the precursor solution suggested a decrease in crystallite size. The lattice parameters for samples of CuO powder (a≠b≠c, α = γ = 90°≠β for monoclinic structure) have been calculated using the relation:
| (2) |
where, d is the inter planar spacing, h, k, l are Miller indices of the crystal planes, a, b, c, β are the lattice parameters. The values of the lattice parameters are recorded in Table 2.
FESEM and HRTEM analysis: The FESEM and HRTEM images of both CuO samples (NP1 and NP2) were taken and analyzed. The NP1 sample showed a greater extent of agglomeration as compared to NP2. The decrease in agglomeration in case of NP2 (prepared with high pH value) may be attributed to the large quantity of the gas evolved. Moreover, NP2 sample also shows a sharp particle distribution and low agglomeration.
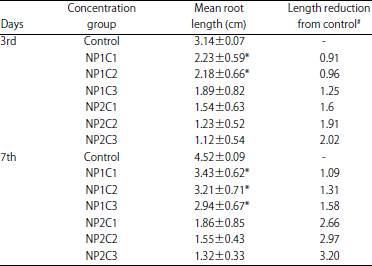
Root length measurement: Selected 5 onion bulbs were taken for the root length measurement. The root length was measured on 3rd and 7th day using a calibrated ruler. Table 3 shows the mean root lengths among different concentration groups of CuO nano-particles.
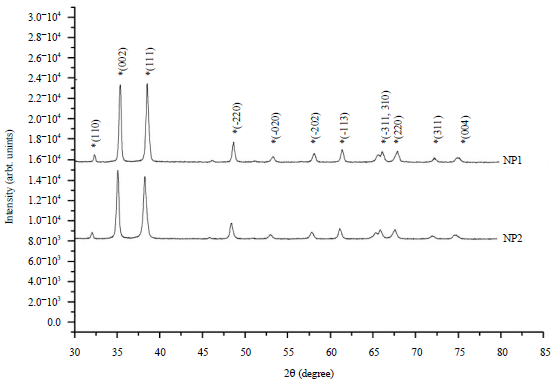 | |
| Fig. 1: | XRD spectrum of CuO samples synthesized at pH7: NP1 and pH10: NP2 |
| Table 2: | Values of the lattice parameters and crystallite size calculated from X-ray diffraction analysis and TEM measurement for NP1 and NP2 samples |
 | |
| Table 3: | Mean root lengths and length reduction from control among different concentration groups of CuO nano-particles synthesized at pH 7 (NP1) and pH 10 (NP2) |
 | |
| *p<0.05, as compared to corresponding concentration group at same day, #Average control root length, 3rd day: 3.14 cm, 7th day: 4.52 cm | |
3rd day measurements: At 3rd day, the mean root length in control group was found to be 3.14±0.07 cm. In the exposure groups, the maximum mean root length acquired was seen in group NP1C1 (2.23±0.59 cm) (Fig. 2a). When compared to the control mean root length (2.23±0.59 vs. 3.14±0.07 cm) a reduction of 0.91 cm was found among NP1C1 group. Similarly, a reduction in mean root length was found to be 0.96 and 1.25 cm in NP1C2 and NP1C3 groups, respectively (Table 3).
Figure 2b shows the comparative mean root lengths among three concentration groups of CuO nano-particles synthesized at pH10 of the precursor solution (NP2). It was realized that the mean root length decreased with increased treatment concentration per group. At 3rd day, maximum mean root length in NP2 group was found to be 1.54±0.63 cm with a mean reduction of 1.6 cm as compared to the control group. Right side of Fig. 3 shows the increased reduction in the mean root lengths among NP2 exposed group in comparison to NP1 group at day 3rd. It is clearly demonstrated that at 3rd day, the reduction in the mean root lengths at different concentrations was higher in NP2 group as compared to NP1 (2.02 vs. 1.25 cm, at highest concentration; Fig. 2a, b). This revealed more toxic nature of NP2 group nano-particles which were synthesized at pH10 as compared to nano-particles synthesized at pH 7.
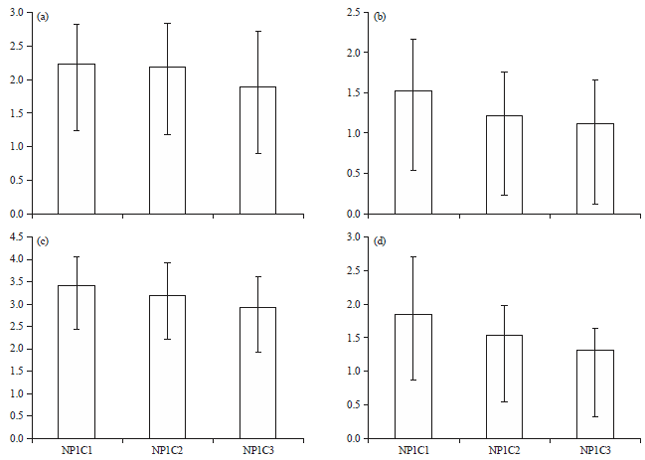 | |
| Fig. 2(a-d): | Comparative bar graphs showing mean root lengths (cm) among different groups of exposed samples, (a) Mean root lengths among three treatment concentration groups of NP1 at day 3rd, (b) Mean root lengths among three treatment concentration groups of NP2 at day 3rd, (c) Mean root lengths among three treatment concentration groups of NP1 at day 7th and (d) Mean root lengths among three treatment concentration groups of NP2 at day 7th |
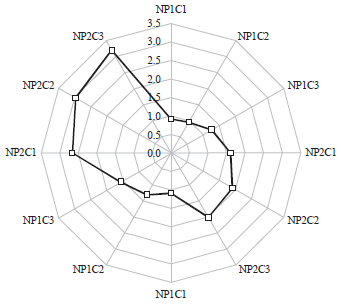 | |
| Fig. 3: | Reduction in the mean root length (cm) after exposure to different concentrations of CuO nano-particles synthesized at pH 7 (NP1) and pH 10 (NP2). Right side denotes 3 days exposure and left side denotes a 7 days exposure |
7th day measurements: At 7th day, the mean root length in control group was found to be 4.52±0.09 cm. Maximum mean root length was found in group NP1C1 (3.43±0.62 cm) (Fig. 2c) among the exposed groups. A reduction of 1.09 cm was found among NP1C1 group in comparison to the control mean root length (3.43±0.62 vs. 4.52±0.09 cm). Similarly, at 7th day, a reduction of mean root length in NP1C2 and NP1C3 groups was found to be 1.31 and 1.58 cm, respectively (Fig. 2c, 3).
Figure 2d shows the comparative mean root lengths among three concentration groups of CuO nano-particles synthesized at pH 10 of the precursor solution (NP2). The mean root length decreased with increase in the treatment concentration per group. At 7th day, maximum mean root length in NP2 group was found to be 1.86±0.85 cm with a mean reduction of 2.66 cm as compared to the control group. Left side of Fig. 3 shows the increased reduction in the mean root lengths among NP2 exposed group in comparison to NP1 group at day 7th. It is clearly demonstrated that at 7th day, the reduction in the mean root lengths at different concentrations was higher in NP2 group as compared to NP1 (3.20 vs. 1.58 cm at highest concentration (Fig. 2c, d)). Thus, at 7th day, higher toxicity of NP2 group nano-particles was recorded which were synthesized at pH 10 as compared to NP1 group.The results of the study clearly demonstrated a higher toxic nature of CuO nano-particles synthesized at pH 10 (NP2 group) as compared to NP1 group synthesized at pH 7. The increased toxicity of NP2 group may be attributed to decreased agglomeration as well as smaller particle size as revealed by SEM, TEM and XRD analysis. Similarly, a study by Hsueh et al.19 reported the effect of pH of the medium on toxicological properties of CuO nano-particles. In this study CuO nano-particles exhibited significant toxicities at pH 5 against four different strains of Staphylococcus aureus strains, including Newman, SA113, USA300 and ATCC6538. At this particular pH (not at pH 6 and 7), CuO nano-particles effectively caused reduction of SA113 and Newman cells and a 20 mM concentration, killed most of the strains. The nano-particles were found to be more soluble at pH 5 than at pH 6 and 7 and the toxicity of CuO nano-particles in mildly acidic pH 5 is caused by Cu2+ release. Similarly, another study reported a retarded growth in onion roots treated with CuO nano-particles after 24 h treatment in comparison to control20.
Some other studies also reported similar results. The surface of the root cap and meristematic zone were found to be damaged by CuO nano-particle exposure. The apical meristem of roots treated by 10 mg L–1 and above concentrations stopped cellular divisions20. Another study reported a gradual decline in mitotic index and increase in abnormality index as the concentration of copper nano-particles and treatment duration were increased8. The ZnO NPs were also found to cause a concentration-dependent inhibition of root length in Allium sativum. When treated with 50 mg L–1 ZnO nano-particles for 24 h, the root growth of garlic was found to be completely blocked21. The CuO nano-particles have also been shown to affect morphology of Landoltia punctata22. The CuO nano-particles can interact with arsenic to affect lengths and biomasses of seedling shoots and roots and on root branching in Oryza sativa japonica 'Koshihikari'23. A reduced germination rate, root and shoot length and biomass was observed at high concentrations of CuO nano-particles24. Bulk- and nano-particles of copper have been found as highly phytotoxic to Cucurbita pepoalso25. Overall growth and transpiration were reduced by 60-70% in comparison to untreated controls. Future studies are highly recommended to evaluate the toxic effects of CuO nano-particles using different models so as to complete their toxic profile. Knowledge about the effects of pH on morphological and toxicological properties of CuO nano-particles may help in formulating future nano-particle safety programs.
Toxicity of CuO nano-particles synthesized at pH7 and pH10 was evaluated using Allium cepa root analysis test. Nano-particles synthesized at pH 10 were found to be smaller in size as compared to particles synthesized at pH 7, as revealed by SEM, TEM and X-ray diffraction analysis. The CuO nano-particles showed a dose dependent toxicity at 3rd and 7th day with a significant reduction in mean root length in the treated onion bulbs as compared to the controls (0.1 g/100 mL, 3rd day: CuO pH7, 1.89±0.82 cm and CuO pH 10, 1.12±0.54 vs. control, 3.14±0.07 cm, p<0.05) and 0.1 g/100 mL, 7th day: CuO pH 7, 2.94±0.67 cm and CuO pH 10, 1.32±0.33 vs. control, 4.52±0.09 cm, p<0.05). The CuO nano-particles synthesized at pH 10 were found to be more toxic as compared to pH 7 samples. Thus, pH may play a vital role in determining the particle size of the synthesized nano-particles, which in-turn decides the toxicity.
This study revealed the effect of pH of the precursor solution on copper oxide nano-particles during their synthesis. Precursor solution pH may alter their morphological and toxicological properties as shown by SEM, TEM, XRD and root analysis test. The present paper can be beneficial for researchers working on effect of pH on nano-particle synthesis and nano-toxicology. Knowing the effects of pH on morphological and toxicological properties of CuO nano-particles may help in formulating future nano-particle safety programs.
The author thanks Dr. Iqbal Singh, Assistant Professor, Department of Physics, Khalsa College, Amritsar for providing nano-particle samples. Author also thanks Dr. Pramjit Singh, Head, Undergraduate Programmes, Segi University, Malaysia, for his constant support throughout the work.