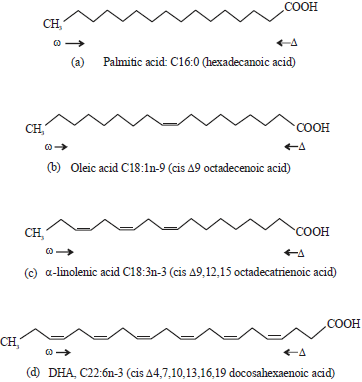
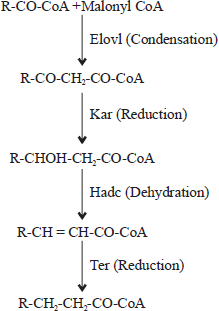
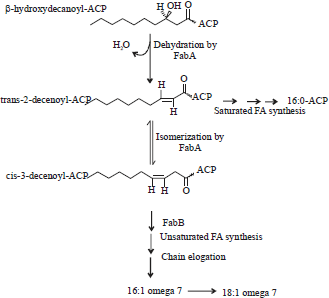
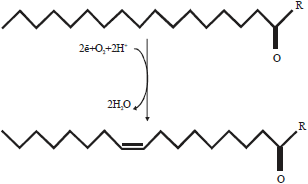
Review Article
Review on Fatty Acid Desaturases and their Roles in Temperature Acclimatisation
Enzyme and Microbial Technology Research Centre, Faculty of Biotechnology and Biomolecular Sciences, University Putra Malaysia, 43400 UPM Serdang, Selangor Darul Ehsan, Malaysia
Mohd Shukuri Mohamad Ali
Enzyme and Microbial Technology Research Centre, Faculty of Biotechnology and Biomolecular Sciences, University Putra Malaysia, 43400 UPM Serdang, Selangor Darul Ehsan, Malaysia
Siti Nurbaya Oslan
Enzyme and Microbial Technology Research Centre, Faculty of Biotechnology and Biomolecular Sciences, University Putra Malaysia, 43400 UPM Serdang, Selangor Darul Ehsan, Malaysia
Raja Noor Zaliha Raja Binti Abdul Rahman
Enzyme and Microbial Technology Research Centre, Faculty of Biotechnology and Biomolecular Sciences, University Putra Malaysia, 43400 UPM Serdang, Selangor Darul Ehsan, Malaysia