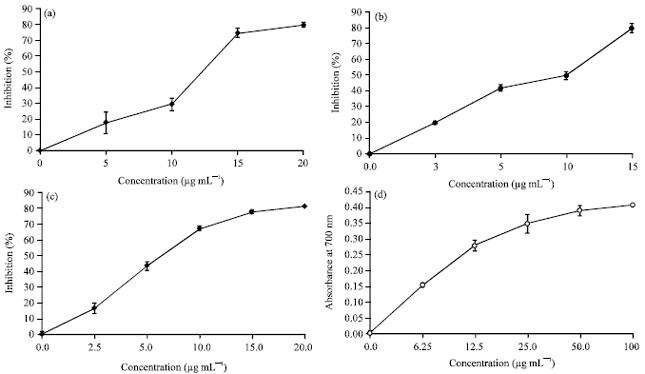
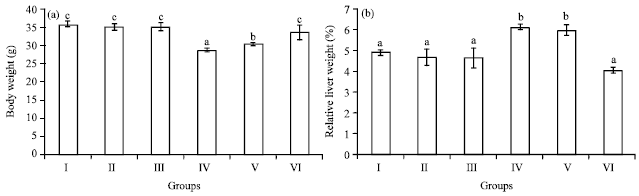
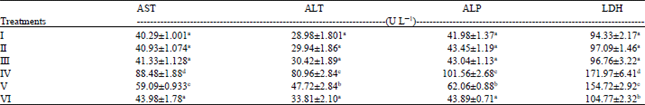
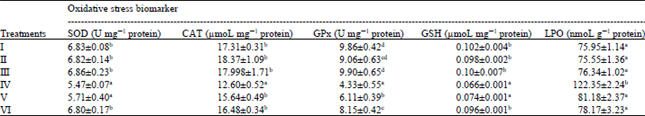
Research Article
Antioxidant Potential and Hepatoprotective Activity of Origanum majorana Leaves Extract against Oxidative Damage and Hepatotoxicity Induced by Pirimiphos-Methyl in Male Mice
Environmental Toxicology Research Unit (ETRU), Department of Pesticide Chemistry, National Research Centre, Tahrir Street, P.O. Box 12311, Dokki, Cairo, Egypt
Tarek M. Heikal
Environmental Toxicology Research Unit (ETRU), Department of Pesticide Chemistry, National Research Centre, Tahrir Street, P.O. Box 12311, Dokki, Cairo, Egypt
Samia M.M. Mohafrash
Environmental Toxicology Research Unit (ETRU), Department of Pesticide Chemistry, National Research Centre, Tahrir Street, P.O. Box 12311, Dokki, Cairo, Egypt
Amel A. Refaie
Environmental Toxicology Research Unit (ETRU), Department of Pesticide Chemistry, National Research Centre, Tahrir Street, P.O. Box 12311, Dokki, Cairo, Egypt