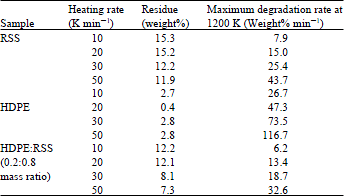
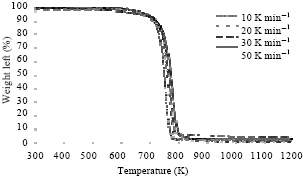
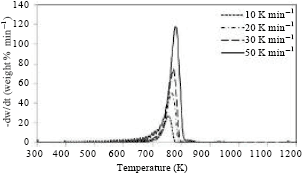
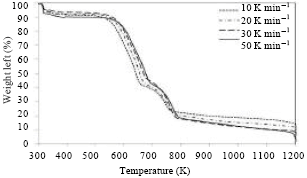
Research Article
Thermal Degradation Behaviour for Co-gasification of Rubber Seed Shell and High Density Polyethylene Mixtures using Thermogravimetric Analysis
Biomass Processing Lab, Centre for Biofuel and Biochemical Research, Green Technology MOR, Department of Chemical Engineering, Universiti Teknologi Petronas, Tronoh 31750, Perak Darul Ridzuan, Malaysia
S. Yusup
Biomass Processing Lab, Centre for Biofuel and Biochemical Research, Green Technology MOR, Department of Chemical Engineering, Universiti Teknologi Petronas, Tronoh 31750, Perak Darul Ridzuan, Malaysia
A. Al Shoaibi
Department of Chemical Engineering, The Petroleum Institute, P.O. Box 2533, Abu Dhabi, United Arab Emirates
S.A. Sulaiman
Department of Mechanical Engineering, Universiti Teknologi Petronas, Tronoh, 31750, Perak Darul Ridzuan, Malaysia