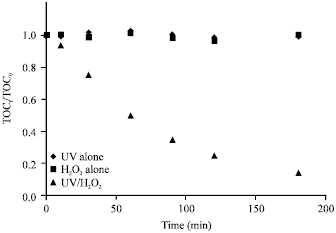
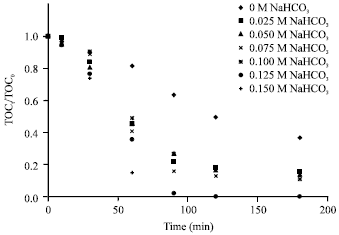
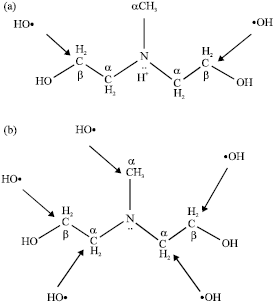
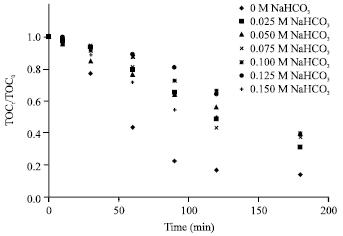
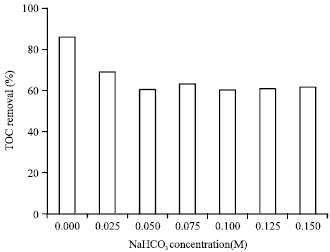
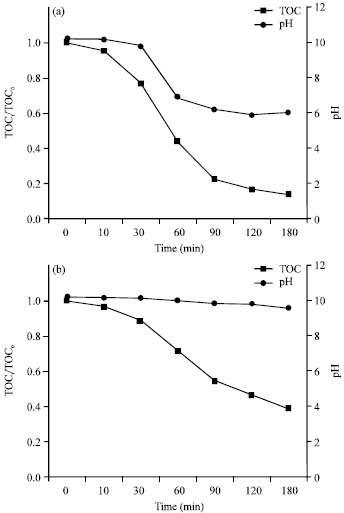
Research Article
Effect of Bicarbonate on the Mineralization of Methyldiethanolamine by using UV/H2O2
Department of Chemical Engineering, Universiti Teknologi PETRONAS, Bandar Seri Iskandar, Tronoh, 31750, Perak Darul Ridzwan, Malaysia
Anisa Ur Rahmah
Department of Chemical Engineering, Universiti Teknologi PETRONAS, Bandar Seri Iskandar, Tronoh, 31750, Perak Darul Ridzwan, Malaysia
Abdul A. Omar
Department of Chemical Engineering, Universiti Teknologi PETRONAS, Bandar Seri Iskandar, Tronoh, 31750, Perak Darul Ridzwan, Malaysia
Thanapalan Murugesan
Department of Chemical Engineering, Universiti Teknologi PETRONAS, Bandar Seri Iskandar, Tronoh, 31750, Perak Darul Ridzwan, Malaysia