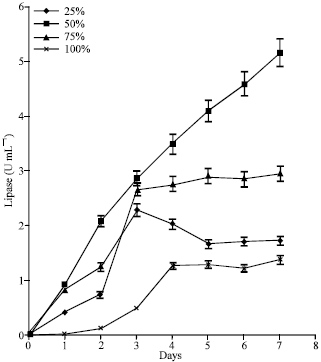
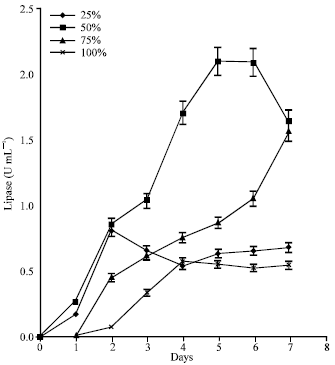
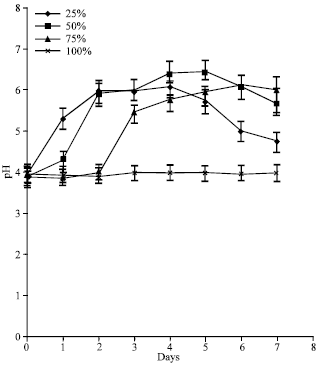
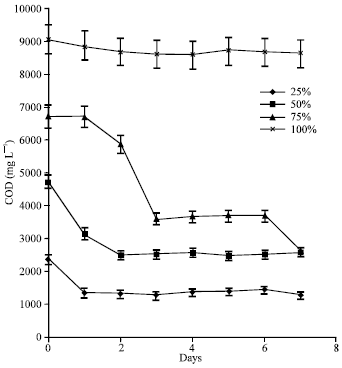
Research Article
Lipase Production from Palm Oil Mill Effluent by Aspergillus terreus Immobilized on Luffa Sponge
Department of Microbiology, University of Nigeria, Nsukka, Nigeria
Hideki Aoyagi
Department of Bioscience and Bioengineering, Graduate School of Life and Environmental Sciences, University of Tsukuba, Ibaraki, Japan
James C. Ogbonna
Department of Microbiology, University of Nigeria, Nsukka, Nigeria