Research Article
3D Surface Characterization of Electrophoretic Deposition Assisted Polishing of SS316L
Department of Mechanical Engineering, Dr. Babasaheb Ambedkar Technological University, Lonere-402 103, Raigad, MS, India
Yogesh Gaikhe
Department of Mechanical Engineering, Dr. Babasaheb Ambedkar Technological University, Lonere-402 103, Raigad, MS, India
Sandeep Huddedar
Department of Mechanical Engineering, Dr. Babasaheb Ambedkar Technological University, Lonere-402 103, Raigad, MS, India
Raju Pawade
Department of Mechanical Engineering, Dr. Babasaheb Ambedkar Technological University, Lonere-402 103, Raigad, MS, India










ajat sudrajat Reply
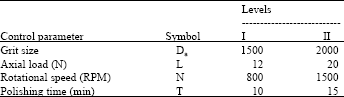
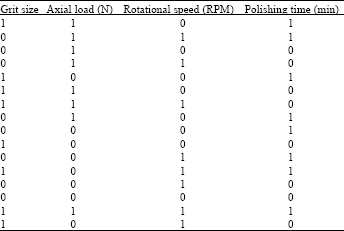
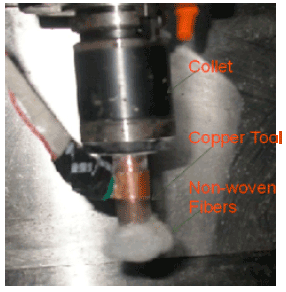
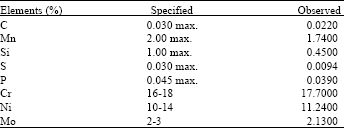
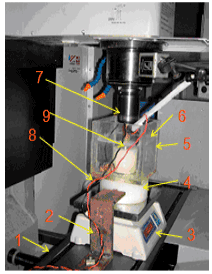
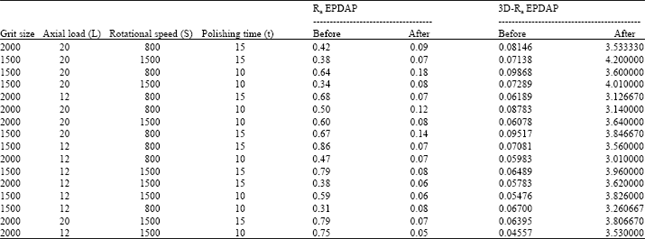
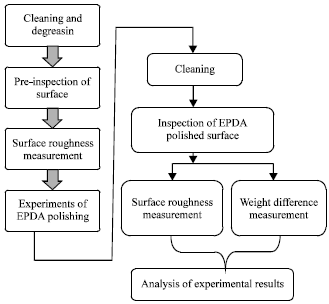
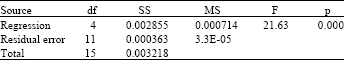
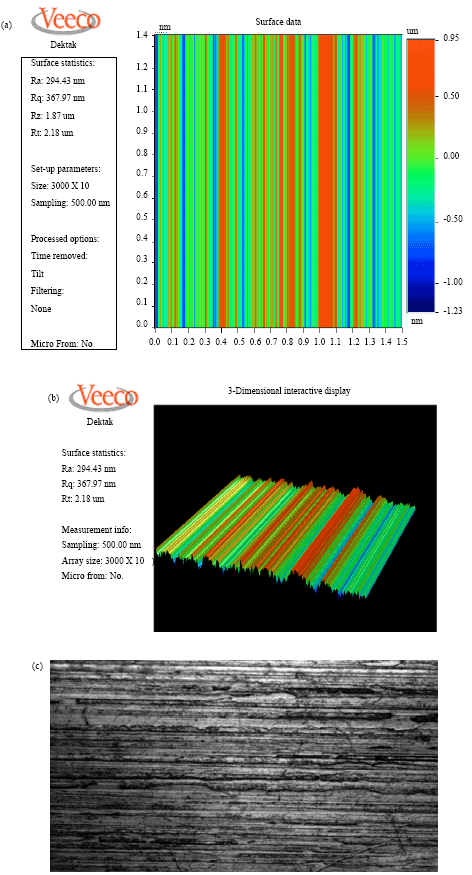
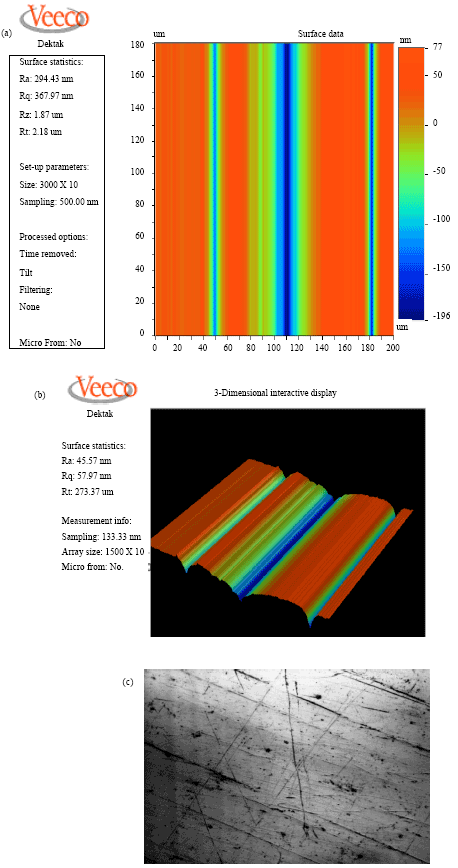
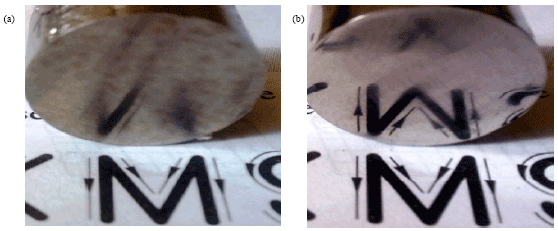
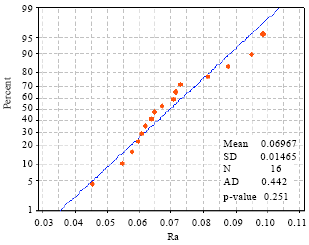
effect of surface quality roughness of SS316L