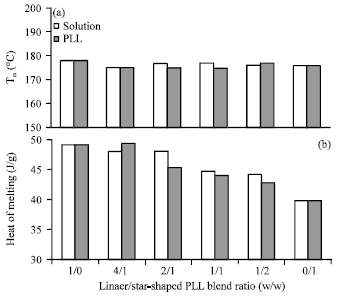
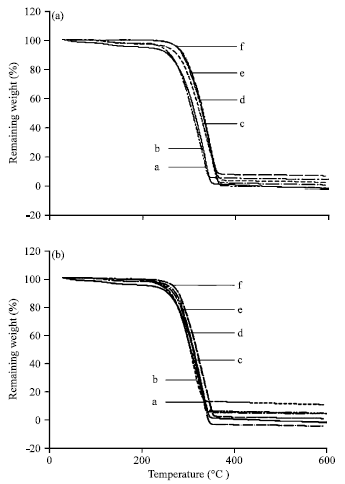
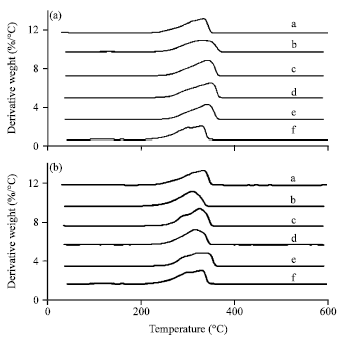
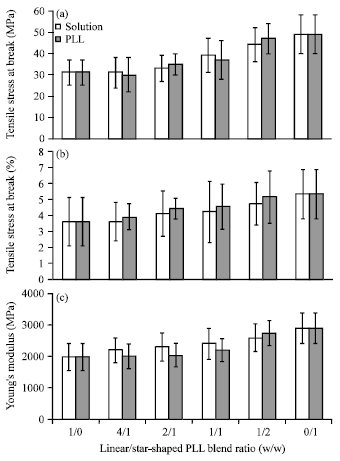
Research Article
Biodegradable Linear/Star-shaped Poly(L-lactide) Blends Prepared by Single Step Ring-opening Polymerization
Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Mahasarakham University, Mahasarakham 44150, Thailand
Yaowalak Srisuwan
Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Mahasarakham University, Mahasarakham 44150, Thailand