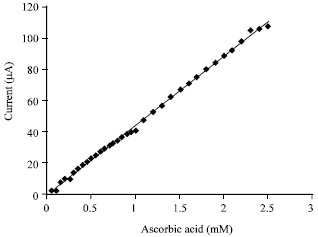
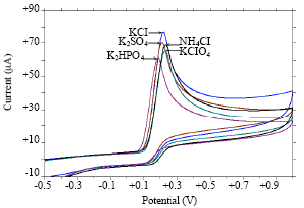
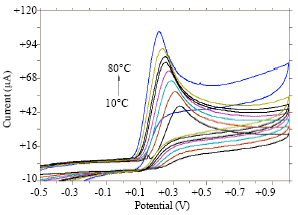
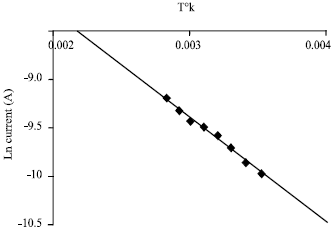
Research Article
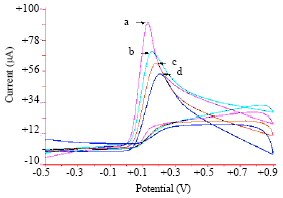
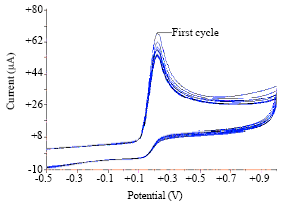
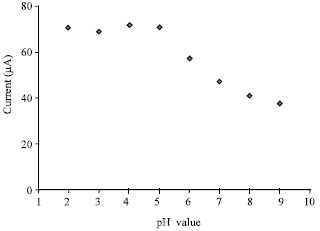
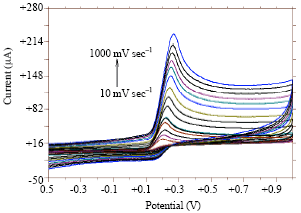
Voltammetric Oxidation of Ascorbic Acid Mediated by Multi-Walled Carbon Nanotubes/Titanium Dioxide Composite Modified Glassy Carbon Electrode
Department of Chemistry, Universiti Putra Malaysia, 43400 Serdang, Selangor D.E., Malaysia
W. T. Tan
Department of Chemistry, Universiti Putra Malaysia, 43400 Serdang, Selangor D.E., Malaysia
N. A. Yusof
Department of Chemistry, Universiti Putra Malaysia, 43400 Serdang, Selangor D.E., Malaysia
J. K. Goh
School of Science, Monash University, 46150 Bandar Sunway, Selangor D.E., Malaysia