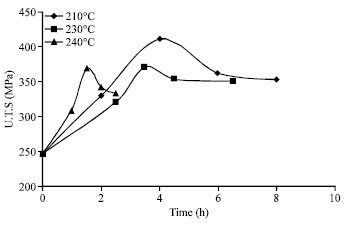
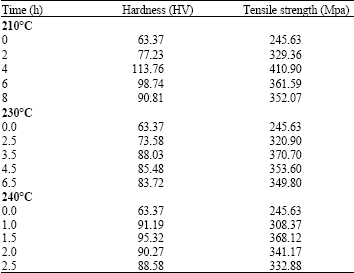
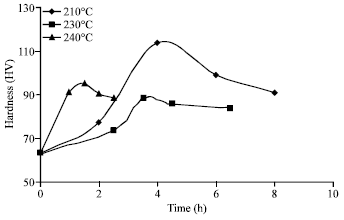
Research Article
Development and Strengthening of 2219 Aluminium Alloy by Mechanical Working and Heat Treatment
Department of Mechanical Engineering, University Technology PETRONAS, Bandar Sri Iskandar, Tronoh 31750 Perak, Malaysia
Faiz Ahmad
Department of Mechanical Engineering, University Technology PETRONAS, Bandar Sri Iskandar, Tronoh 31750 Perak, Malaysia
N. Ikram
Centre of Excellence in Solid State Physics, University of the Punjab, Pakistan
R. Ahmad
Faculty of Engineering and Technology, University of the Punjab, Quaid-e-Azam Campus, Lahore-54590 Punjab, Pakistan
A. Salam
Faculty of Engineering and Technology, University of the Punjab, Quaid-e-Azam Campus, Lahore-54590 Punjab, Pakistan