Research Article
New Technique for Studying Soil-Corrosion of Underground Pipeline
Reliability Engineering and Safety Assessment Research Group (RESA), Department of Structure and Material, Faculty of Civil Engineering, Universiti Teknologi Malaysia
Norhazilan Md Noor
Reliability Engineering and Safety Assessment Research Group (RESA), Department of Structure and Material, Faculty of Civil Engineering, Universiti Teknologi Malaysia
Siti Rabe`ah Othman
Reliability Engineering and Safety Assessment Research Group (RESA), Department of Structure and Material, Faculty of Civil Engineering, Universiti Teknologi Malaysia
Lim Kar Sing
Reliability Engineering and Safety Assessment Research Group (RESA), Department of Structure and Material, Faculty of Civil Engineering, Universiti Teknologi Malaysia
Mazura Mat Din
Faculy of Computer Science and Information Technology, Universiti of Teknologi Malaysia, 81310, Skudai, Johor, Malaysia











Evans, U Felix Reply
I wish to join your research team, your ideas are quite good.
Hawkar, J Muhammed Reply
Dear sir
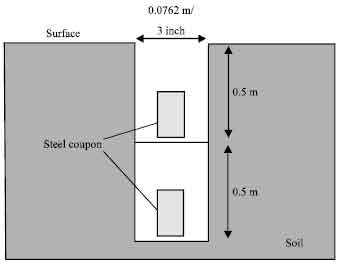
I hope you are doing well, I would like to ask about the criteria or standard that have chosen to select the size of coupon that buried in soil.
thanks for your help
Beast regards