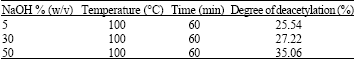Research Article
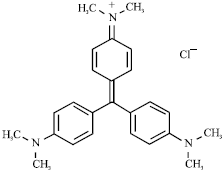
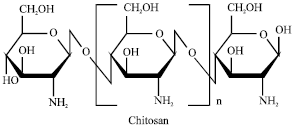
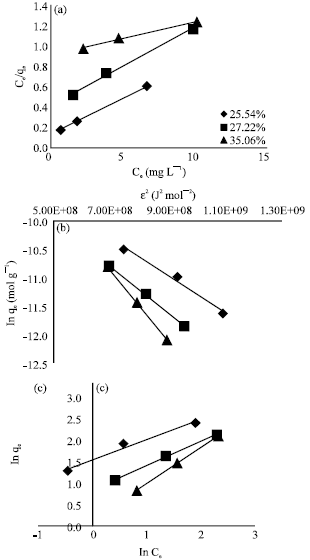
Removal of A Cationic Dye using Deacetylated Chitin (Chitosan)
Industrial Chemistry Programme, School of Science and Technology, Universiti Malaysia Sabah, 88999 Kota Kinabalu, Sabah, Malaysia
C. Y. Yee
Industrial Chemistry Programme, School of Science and Technology, Universiti Malaysia Sabah, 88999 Kota Kinabalu, Sabah, Malaysia
H. S. Eng
Industrial Chemistry Programme, School of Science and Technology, Universiti Malaysia Sabah, 88999 Kota Kinabalu, Sabah, Malaysia