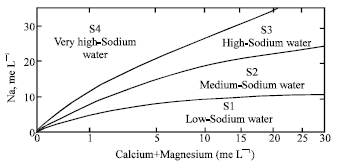
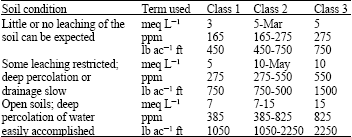
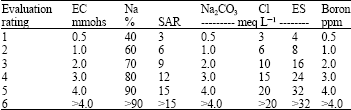
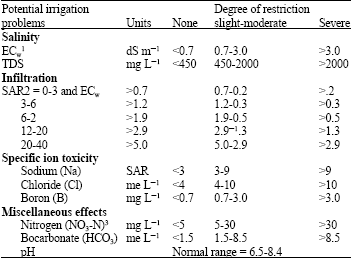
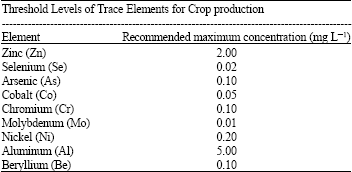
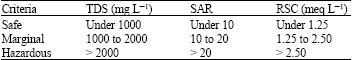
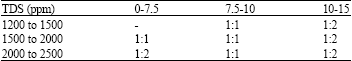
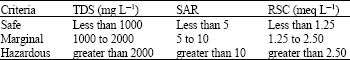Research Article
Guidelines for Irrigation Water Quality and Water Management in The Kingdom of Saudi Arabia: An Overview
National Center for Water Research, King Abdulaziz City for Science and Technology, P.O. Box 6086, Riyadh, 11442, Kingdom of Saudi Arabia
A. Alquwaizany
National Center for Water Research, King Abdulaziz City for Science and Technology, P.O. Box 6086, Riyadh, 11442, Kingdom of Saudi Arabia
A. Al-Zarah
National Center for Water Research, King Abdulaziz City for Science and Technology, P.O. Box 6086, Riyadh, 11442, Kingdom of Saudi Arabia