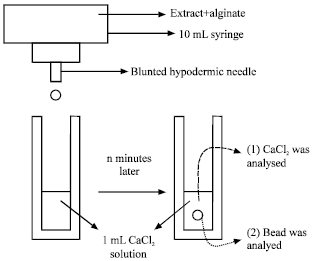
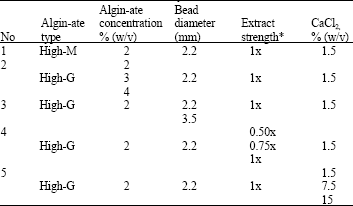
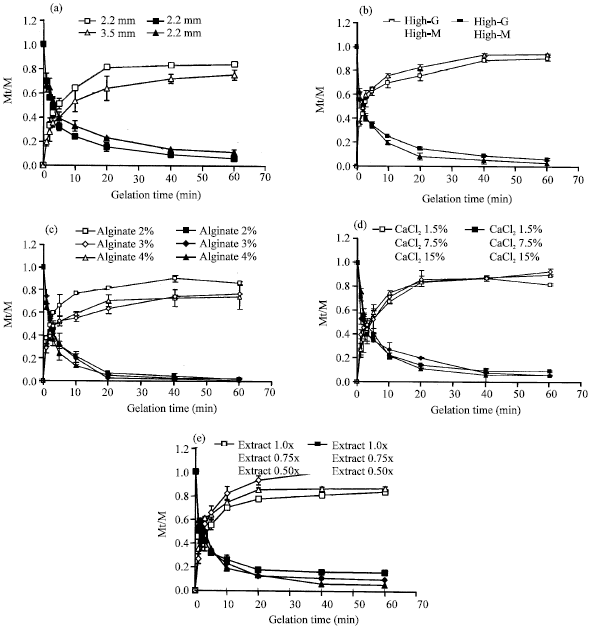
Research Article
Release Kinetics of Encapsulated Herbal Antioxidants during Gelation Process
Centre of Materials and Minerals, School of Engineering and IT, Universiti Malaysia Sabah, 88999 Kota Kinabalu, Sabah, Malaysia
C.B. Tiong
Centre of Materials and Minerals, School of Engineering and IT, Universiti Malaysia Sabah, 88999 Kota Kinabalu, Sabah, Malaysia
R.F. Mansa
Centre of Materials and Minerals, School of Engineering and IT, Universiti Malaysia Sabah, 88999 Kota Kinabalu, Sabah, Malaysia
P. Ravindra
Centre of Materials and Minerals, School of Engineering and IT, Universiti Malaysia Sabah, 88999 Kota Kinabalu, Sabah, Malaysia
E.S. Chan
Centre of Materials and Minerals, School of Engineering and IT, Universiti Malaysia Sabah, 88999 Kota Kinabalu, Sabah, Malaysia