Research Article
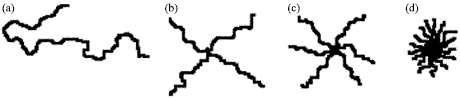
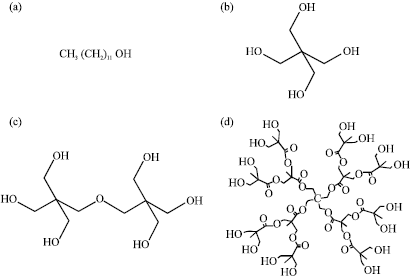
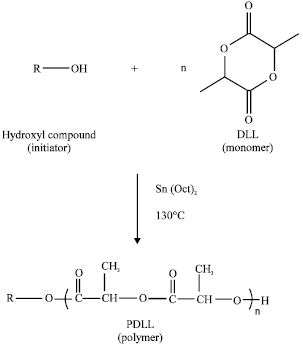
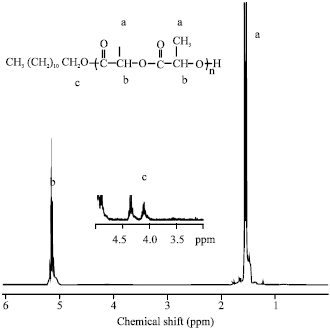
Effects of Arm Number and Arm Length on Thermal Properties of Linear and Star-shaped Poly(D,L-lactide)s
Center of Excellence for Innovation in Chemistry, Department of Chemistry, Faculty of Science, Mahasarakham University, Mahasarakham 44150, Thailand
Yodthong Baimark
Center of Excellence for Innovation in Chemistry, Department of Chemistry, Faculty of Science, Mahasarakham University, Mahasarakham 44150, Thailand