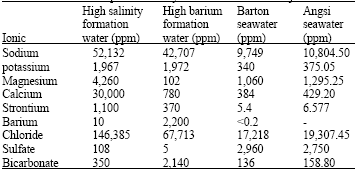
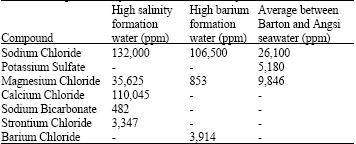
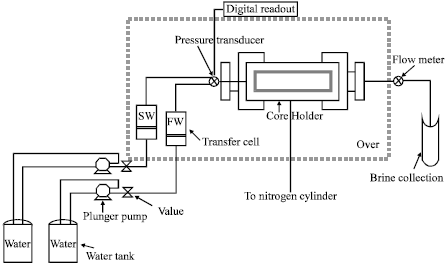
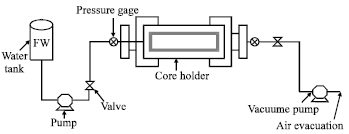
Research Article
Scale Formation Due to Water Injection in Berea Sandstone Cores
Faculty of Chemical and Natural Resources Engineering, Universiti Teknologi Malaysia, 81310 Skudai, Johor, Malaysia
A.A.M. Yassin
Faculty of Chemical and Natural Resources Engineering, Universiti Teknologi Malaysia, 81310 Skudai, Johor, Malaysia