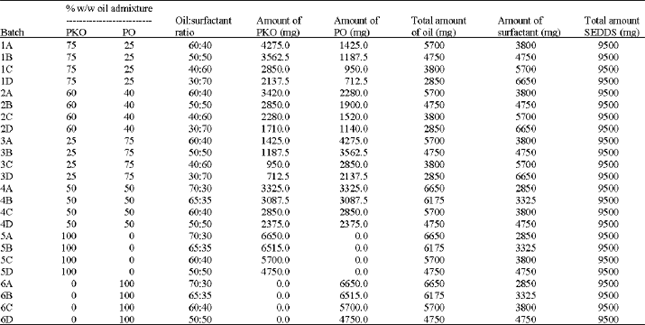
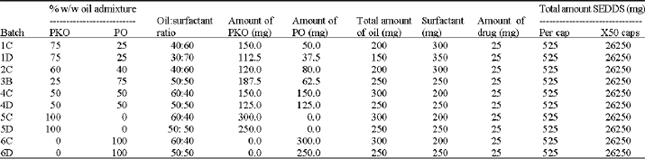
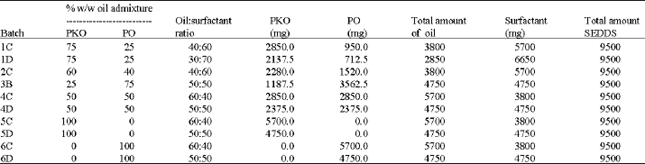
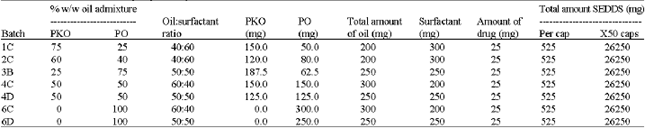
Research Article
Preliminary Studies on Two Vegetable Oil Based Self Emulsifying Drug Delivery System (SEDDS) for the Delivery of Metronidazole, A Poorly Water Soluble Drug
Department of Pharmaceutical Technology and Industrial Pharmacy, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Nigeria
H. Ezeiruaku
Department of Pharmaceutical Technology and Industrial Pharmacy, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Nigeria
V.I. Onyishi
Department of Pharmaceutical Technology and Industrial Pharmacy, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Nigeria