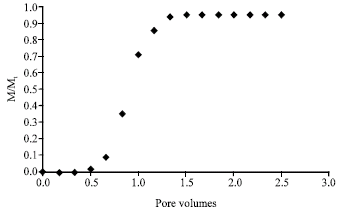
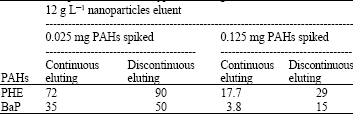
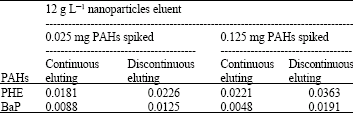
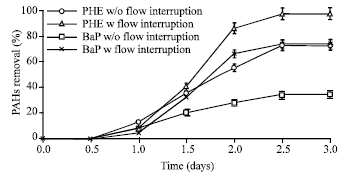
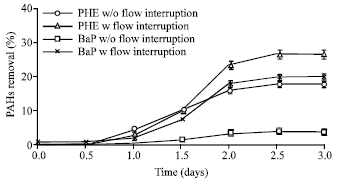
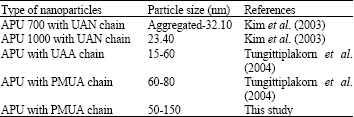
Research Article
A Preliminary Study for Removing Phenanthrene and Benzo(a) Pyrene from Soil by Nanoparticles
Environmental Research Institute, Chulalongkorn University, Phayathai Rd, Patumwan, Bangkok 10330, Thailand
Onarpar Santisukkasaem
National Center of Excellence for Environmental and Hazardous Waste Management,Chulalongkorn University, Phayathai Rd, Patumwan, Bangkok 10330, Thailand