Research Article
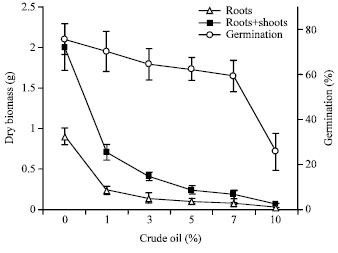
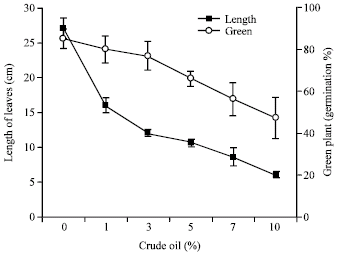
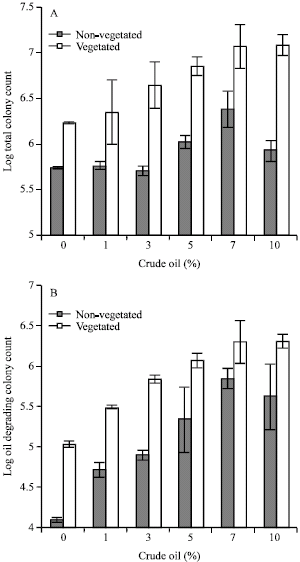
Effect of Light Crude Oil-Contaminated Soil on Growth and Germination of Festuca arundinacea
Laboratory of BioResearch, Department of Biology, Faculty of Sciences, Shahid Beheshti University, Tehran, Iran
Malek-Hossein Shahriari
Department of Soil Science Engineering, College of Agriculture and Natural Resources, Tehran University, Karaj, Iran
Gholamreza Savaghebi -Firoozabadi
Department of Soil Science Engineering, College of Agriculture and Natural Resources, Tehran University, Karaj, Iran
Faeze Kalantari
Laboratory of BioResearch, Department of Biology, Faculty of Sciences, Shahid Beheshti University, Tehran, Iran
Mahdie Anzi
Laboratory of BioResearch, Department of Biology, Faculty of Sciences, Shahid Beheshti University, Tehran, Iran