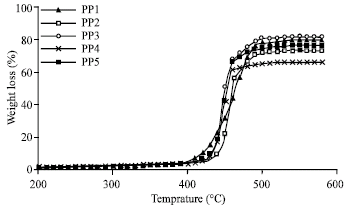
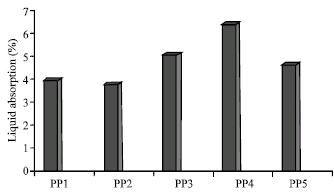
Research Article
Thermal Stability, Mechanical Properties and Solvent Resistance of PP/clay Nanocomposites Prepared by Melt Blending
Department of Chemical Engineering, Tehran University, Tehran, Iran
Seyed Javad Ahmadi
Jaber Ebne Hayyan Research Laboratories, Atomic Energy Organization of Iran, P.O. Box 11365 8486, Tehran, Iran
Hossein Abolghasemi
Department of Chemical Engineering, Tehran University, Tehran, Iran
Ahmad Mohaddespour
Department of Chemical Engineering, Tehran University, Tehran, Iran